Volume 9, Issue 3 (Summer 2021)
PCP 2021, 9(3): 227-236 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohajeri Aval N. The Effectiveness of Transcranial Direct Current Stimulation (tDCS) on Improving the Severity of Stuttering and Anxiety in School-aged Children Who Stutter. PCP 2021; 9 (3) :227-236
URL: http://jpcp.uswr.ac.ir/article-1-747-en.html
URL: http://jpcp.uswr.ac.ir/article-1-747-en.html
Department of Psychology, Faculty of Educational Science and Psychology, Karaj Branch, Islamic Azad University, Alborz, Iran. , nastaranmohajeri@yahoo.com
Full-Text [PDF 709 kb]
(1916 Downloads)
| Abstract (HTML) (4597 Views)
Full-Text: (1339 Views)
1. Introduction
Developmental stuttering is a neurodevelopmental condition disrupting the smooth flow of speech, resulting in characteristic speech disfluencies. Developmental stuttering has been associated with reduced educational and employment opportunities (Klein & Hood , 2004), social anxiety (Iverach et al., 2009), and compromised quality of life (Craig, Blumgart, & Tran, 2009). The fluency of speech is interrupted by moments of stuttering, which include repetitions and prolongation of speech sounds and ‘blocks’ during which speech sounds cannot be produced (Chesters, Mottonen, & Watkins, Smith, Davis, & Howell, 2018).
Stuttering is accompanied by numerous negative consequences across the lifespan, which may increase vulnerability to social and psychological challenges (Schneier, Wexler, & Liebowitz, 1997). These negative consequences of stuttering result in the development of anxiety (Blood & Blood, 2007). Empirical studies of anxiety and stuttering have mostly supported a positive relationship between them (Kraaimaat, Vanryckeghem, & Van Dam-Baggen, 2002). In addition, a positive relationship between the severity of stuttering and the level of anxiety has been reported (Caruso, Chodzko-Zajko, Bidinger, & Sommers, 1994; Fitzgerald, Djurdjic, & Maguin, 1992). Children who stutter may begin to show impaired behavioral, emotional, and social development as early as age 3, and these difficulties are established in older children who stutter (McAlister, 2016).
People Who Stutter (PWS) show subtle abnormalities in the structure and function of the brain regions supporting speech. In particular, the Inferior Frontal Cortex (IFC) is consistently highlighted as the affected region. The IFC plays a crucial role in speech production, comprising parts involved in motor planning and integration of sensory signals (Bohland, Bullock, & Guenther, 2010; Hickok & Poeppel, 2007). Watkins et al. showed that a portion of left IFC – the ventral premotor cortex – was under-active during speaking and that the white matter underlying this region was disrupted (Watkins et al., 2008).
Scientists have been studying the effect of direct current stimulation since the early 1900s (Bindman, Lippold, & Redfearn,1964; Elsberg 1917; Fuortes 1954; Hern, J. E., Landgren, Phillips, & Porter, 1962). Nevertheless, this research technique was ignored for all intents and purposes for several decades. In the late 1990s, an interest in the transcranial Direct Current Stimulation (tDCS) effect on the human central nervous system re-emerged (Jacobson, Koslowsky, & Lavidor, 2012). Stuttering status and persistence are associated with aberrant network connectivity involving the default mode network and its connections with attention, somatomotor, and frontoparietal networks (Chang et al., 2018).
tDCS is a noninvasive brain stimulation method, which could improve the outcomes of fluency interventions in people who stutter (Chesters, Watkins, & Mottonen, 2017). tDCS modulates cortical excitability by applying weak electrical currents in the form of Direct Current (DC) brain polarization. Depending on direct current polarity, neuronal firing rates increase or decrease, presumably due to DC-induced changes in resting membrane potentials (Liebetanz, Nitsche, Tergau, & Paulus, 2002; Nitsche et al., 2003b), with anodal tDCS in most settings increasing, and cathodal tDCS decreasing motor-cortical excitability (Nitsche & Paulus, 2000, 2001).
In tDCS, neuroplastic effects emerge a short while after stimulation. The process depends on alterations to glutamatergic and GABAergic activities (Nitsche et al., 2003; Stagg et al., 2009). Like the acute membrane polarization effects, anodal tDCS and cathodal tDCS result in excitability-enhancing and excitability-reducing plasticity, respectively (Nitsche et al., 2003; Nitsche & Paulus, 2000).
Several functional imaging studies demonstrated reduced activity during speech production in Broca’s Area (BA) (i.e., the cortical center for speech production) (Fox et al., 1996; Neumann et al., 2005). Conversely, the right homolog region of Broca’s area showed overactivation; this finding has been highly replicated in several studies, including a recent meta-analysis (Brown, Ingham, Ingham, Laird, & Fox, 2005; Budde, Barron, & Fox, 2014; Belyk, Kraft, & Brown, 2015).
Previous studies have examined the effects of neuronal modulation by tDCS on language processing for healthy and clinical populations. For healthy volunteers, several lines of positive evidence have been reported, including facilitatory effects on language production for words and sentences and language learning. Also, 18–20 clinical tDCS studies have reported facilitation effects on impaired language functions in aphasic patients (Fiori et al., 2011; Marangolo et al., 2013). Stuttering severity significantly modulated the impact of stimulation: active stimulation attenuated the atypically strong association between stuttering severity and right thalamocortical network activity, especially in more severe speakers (Garnett, Chow, Choo and Chang, 2019).
Chesters investigated the effect of 5 sessions of anodal tDCS over the left inferior frontal gyrus during a speech fluency intervention on stuttering. Speech fluency significantly improved in the treatment group that received anodal tDCS combined with the fluency intervention compared to the respective sham tDCS group. Thus, using tDCS simultaneously with fluency training can enhance speech fluency in adults who stutter (Chesters et al., 2018).
These improvements are created and stabilized when tDCS is applied in consecutive daily sessions (Reis et al., 2009; Baker, Rorden, & Fridriksson, 2010). Increasingly, tDCS is being investigated as an adjunctive treatment for acquired disorders of motor, language, and cognitive functions (Baker et al., 2010; Marangolo et al., 2011; Khedr et al., 2013; Allman et al., 2016; Mortensen et al., 2016).
Despite advances in stuttering treatment, significant limitations such as instability of treatment outcomes and lack of long-term results have to be addressed (Chesters et al., 2017). Given the past evidence that tDCS can induce plasticity-like changes in cortical functions that outlast the stimulation period, we anticipate that tDCS will help stabilize intervention-related improvements in speech fluency (Nitsche & Paulus, 2001). Speech restructuring interventions were found to reduce stuttering in adults, however, with various degrees and maintenance of fluency. tDCS reduce stuttering frequency by 22%-27% (Brignell, et al., 2020).
This study aimed to evaluate the effect of tDCS on the severity of stuttering and anxiety in school-aged children. I expected that fluency intervention when used anodal tDCS would result in reduced disfluency (i.e., improved fluency) relative to the same fluency intervention with sham stimulation. My research is the first study to investigate tDCS on the severity of stuttering and anxiety in school-aged children.
2. Materials and Methods
This quasi-experimental study has a pretest-posttest and follow-up design with a control group. The study population was all elementary school students who stutter and live in Tehran City, Iran, in 2019-2020. Twenty-two right-handed children who were native Persian speakers (12 boys and 10 girls; aged 8-12 years; median: 10 years) participated in this study. The sample size was calculated based on formerly established studies and methods (Bazargan, Sarmad & Hejazi, 2007). Hence, based on similar research conducted in the past and considering the cost of testing, the sample was selected. The inclusion criteria were the presence of developmental stuttering and a participant age between 8 and 12 years. The exclusion criteria were any speech and language disorder other than developmental stuttering, personal history of seizures, diagnosis of autism spectrum disorder or attention deficit hyperactivity disorder, and neurological or psychiatric illnesses. These criteria were evaluated by a psychiatrist based on DSM-5. In this study, I delivered 1 mA of anodal tDCS over the left IFC for 20 min per day in 15 consecutive daily sessions. Fluency was assessed 1 and 6 weeks after the 15-day intervention. tDCS was performed by the author of the article, who had a tDCS certificate.
Twenty-two participants were assigned in two groups of experimental and sham-controlled. Before the study, the stuttering in the study children was confirmed by a speech therapist. Thus, every participant met the diagnostic criteria of stuttering in the DSM-5. The Stuttering Severity Instrument, version 4 (SSI-4: Riley 2009), was used as a standardized measure of stuttering symptoms, and the Spence scale was used to assess the anxiety severity. All children were evaluated by SSI-4 and Spence scale (by a speech therapist). All parents of participants in this study complete the treatment consent form with full knowledge and consent. Throughout the study, all the ethical guidelines were observed. The subject, purpose, and procedure of the study were explained to all participants. Furthermore, the research participants were allowed to leave the study whenever desired.
Eleven children in the experimental group underwent 15 sessions (5 sessions per week, based on research done in the past) of anodal tDCS for the left IFC. In the experimental group, participants received 20 min of stimulation at 1 mA using 5*7 cm electrodes during the fluency intervention. The anode was placed over the left inferior frontal cortex (centered on F7 according to the 10-20 EEG electrode placement system) and cathode over the right supra-orbital ridge. This montage was tested in a previous study of speech facilitation (Chesters et al., 2017; (Holland, & Crinion, 2012). Electrode position F7 is centered on Broca’s region, extending posteriorly to cover ventral portions of the premotor and primary motor cortex, where the representation of the articulators is located (Bestmann, de Berker, & Bonaiuto, (2015). Similar electrode placement was used in the sham-controlled group, during which the current was ramped up over 15 s, maintained for 15 s at 1 mA, and ramped down over 15 s at the start of the session. For sham stimulation, I delivered a small current pulse every 55 s throughout 20 min. These sham stimulation parameters were delivered at an ineffective current dosage. The researcher of this study performed this intervention.
Stuttering Severity Instrument, version 4 (SSI-4)
To calculate the stuttering frequency, I used SSI-4 (Riley, 2009), which provides a standardized and norm-referenced index of disfluency. This inventory assesses three domains of stuttering: frequency, duration, and physical concomitants. Speech disfluency was measured by the speech therapist at baseline, before and after the intervention, and at 1 week and 6 weeks post-intervention. The reliability of this test was obtained using the Cronbach α for the whole test as 0.93 (Riley, 2009). Garmatani, Shafiei, Feizi, Salehi, & Howell, (2012) determined the Cronbach coefficient of the whole Persian version of this tool as 0.98.
Spence Children Anxiety Scale (SCAS)
As there is an increased prevalence of anxiety in developmental stuttering (Iverach et al., 2009, 2011), all children completed the Spence Children Anxiety Scale (SCAS; Spence, 1998, quoted in Mousavi, 2007). I wanted to determine whether the tDCS could decrease the anxiety symptoms. The Spence children anxiety scale was developed to assess the severity of anxiety symptoms broadly in line with the dimensions of anxiety disorder proposed by the Diagnostic and statistical manual of mental disorders (DSM-IV). This measure consists of 44 items, of which 38 reflect specific symptoms of anxiety and 6 are positive, filler items to reduce negative response bias. This scale assesses six domains of anxiety: generalized anxiety, panic/agoraphobia, social phobia, separation anxiety, obsessive-compulsive disorder, and physical injury fears. Children are asked to rate the frequency they experience each symptom on a 4-point scale from never=0 to always=3. Anxiety was measured by a speech therapist at baseline, before and after the intervention, and at 1 week and 6 weeks post-intervention. The Cronbach α coefficient was estimated to be 0.92 for the whole instrument and between 0.60 and 0.82 for its subscales (Spence, 1998, quoted in Mousavi, 2007) examined the reliability of the Persian version of this questionnaire. The Cronbach α coefficient was found between 0.62 and 0.89, and six factors were confirmed by confirmatory factor analysis.
In this research, SPSS version 16 was used for data analysis. To analyze the effects of tDCS during the 15-day intervention on speech fluency and anxiety, we entered the change from baseline severity of stuttering and anxiety measured post-intervention into a mixed-model ANOVA. The effect of tDCS on the outcome measure (change from baseline in the SSI-4 score) was assessed using a mixed-model ANOVA with the between-subject factor of group (tDCS, sham) and a within-subjects factor of post-intervention time (baseline, 1 week, 6 weeks). The other outcome measure (change from baseline in Spens) was also assessed using a mixed-model ANOVA with the between-subject factor of group (tDCS, sham) and a within-subjects factor of post-intervention time (baseline, 1 week, 6 weeks). The means of changes from baseline in percentage of disfluent syllables, with 95% Confidence Intervals (CI), were calculated for the tDCS and sham groups separately, along with the differences in these means between the two groups. Cohen’s d was calculated for the effect sizes of the group differences. The author of this article did tDCS, and she was certified to do so.
3. Results
All participants completed the SSI-4 and Spence scale before and after the intervention and were included in all the analyses. The 1-week post-intervention session was carried out about 8 days after intervention (range 6–13 days) and the 6-week session at about 40 days after intervention (range 32–53 days). Table 1 presents the characteristics of study groups before the intervention, which were well matched between the tDCS and sham groups.

As shown in Table 1, in all test subscales in the experimental group, mean scores of stuttering severity in children in the posttest and follow-up (1-week and 6-week) decreased but in the sham group, no such changes were observed. Also, the mean score of anxiety in children in the posttest and follow-up decreased, but in the sham group, no such change was observed. The significance of group differences according to the pretest, posttest, and follow-up (1 and 6 weeks) scores was examined by mixed-model ANOVA.
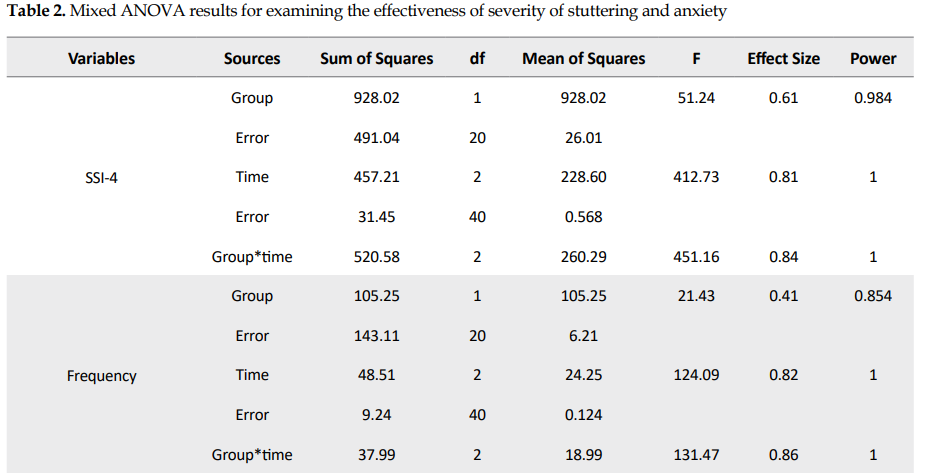
Table 2 presents the results of the analysis of variance of the main effects and their interactions.
.PNG)
.PNG)
As seen in Table 2, analysis of variance result was significant for intragroup (time) and between-group of total SSI-4 factor. The interaction of group effect and time was also significant (F=451.16, df=2). The impact of the intervention in relation to group interaction and time was 0.84, and its statistical power was 1. So, tDCS was effective on the severity of stuttering.
According to Table 2, the analysis of variance result was significant for intragroup and between-group of frequency factor. The interaction of group effect and time was also significant (F=131.47, df=2). The impact of experimental action in relation to group interaction and time was 0.86, and its statistical power was 1. So, tDCS was effective on the frequency factor.
Based on Table 2, the analysis of variance result was significant for intragroup and between-group of duration factor. The interaction of group effect and time was also significant (F=189.31, df=2). The impact of the intervention in relation to group interaction and time was 0.83, and its statistical power was 1. So, tDCS was effective on the duration factor.
Table 2 indicates that the analysis of variance result was significant for intragroup (time) of physical concomitants factor and not significant for between-group factor. These results mean that regardless of the group effect, the effect of time was significant. Also, the interaction of group effect and time was significant (F=144.57, df=2). The effect of the intervention in relation to group interaction and time was 0.84, and its statistical power was 1. So, tDCS was effective on the physical concomitants factor.
Table 2 shows that the analysis of variance result was significant for intragroup and between-group of Spence total factor. The interaction of group effect and time was also significant (F=136.13, df=2). The effect of experimental action in relation to group interaction and time was 0.84, and its statistical power was 1. So, tDCS was effective on the anxiety of children who stutter.
4. Discussion
The present study aimed to examine the effectiveness of tDCS on the severity of stuttering and anxiety of children who stutter. We hypothesized that administration of tDCS to the left IFC could decrease the severity of stuttering and anxiety. Speech fluency and anxiety improved in the experimental group for at least 6 weeks after the intervention. Left IFC was targeted as a critical brain region in speech production with anodal tDCS expected to enhance neuronal excitability. This region was selected for stimulation because it has a crucial role in coordinating the planning and execution of speech movements and is typically less active in PWS than fluent speakers during speech (Chesters et al., 2017).
Our results were consistent with Marangolo et al., (2011) and Allman et al., 2016) results on the effectiveness of tDCS on reducing the severity of stuttering. Their results reflect that the tDCS parameters effectively modulate speech and motor learning (Allman et al., 2016; Marangolo et al., 2011). However, a systematic direct comparison of various tDCS protocols on speech fluency would help gain more information on the potential clinical benefits of this approach (Allman et al., 2016).
Our results are consistent with previous work in aphasia (Holland, & Crinion, 2012; Monti et al., 2013; Sandars, Cloutman, & Woollams, 2016). tDCS could be combined with different behavioral methods for increasing fluency. Previous studies chose tDCS, as they aimed to reinforce fluent speech, and choral speech induces fluency immediately and more successfully and to a greater extent than other methods (Saltuklaroglu, Kalinowski, Robbins, Crawcour, & Bowers, 2009). Temporary fluency enhancements are associated with normalized activity in the left inferior frontal cortex (Wu et al., 1995; Fox et al., 1996; Toyomura, Fujii, & Kuriki, 2011). Of particular relevance to the current trial are two studies showing increased speech motor skill following anodal tDCS over the left IFC (Marangolo et al., 2011, 2013) in two small samples of patients with acquired apraxia of speech (three and eight patients, respectively). The larger sample size of stuttering participants supports the claim that applying anodal tDCS over the left IFC can increase speech motor rehabilitation outcomes (Chesters et al., 2018).
A previous tDCS study of stuttering has also shown that the speech improvement induced by 1 mA of anodal tDCS over the left IFC was maintained for up to 6 weeks after the intervention. Although one study did not report significant speech improvement for the Broca area block in the anodal session, the effect of tDCS may be different between online and offline periods given several neuromodulatory factors induced by stimulation with various time scales (Filmer, Dux, & Mattingley, 2014).
One study showed that anodal TDCS to the primary motor cortex produces facilitation of corticospinal excitability in only approximately three-quarters of young and healthy participants (Wiethoff, Hamada, & Rothwell, 2014). Another study found that tDCS over area V5/MT combined with a 5-day course of reading therapy improved reading speed and fluency in adults with developmental dyslexia, with benefits persisting 1 week after the intervention (Heth, & Lavidor, 2015).
The SSI-4 was included in the trial as a widely recognized standardized clinical measure, which provided complementary information to the primary outcome measure regarding fluency disruptions. Specifically, the percentage of disfluent syllables is a highly sensitive measure of stuttering frequency, whereas the SSI-4 sacrifices some sensitivity (by conversion to scaled scores) but incorporates essential information regarding the duration of stuttered moments and concomitant features, such as tic-like facial or body movements (Chesters et al., 2018). The subscales of the SSI-4 were statistically analyzed separately. However, an inspection of the means showed that reductions were larger in the tDCS group than the sham group for all subscales (frequency, physical concomitants, and duration), except for the physical concomitants subscale, there were few differences between the two groups.
Much research has been done on the effect of tDCS on anxiety, and research has shown that tDCS has been effective in reducing anxiety (Alizadeh Goradel, Pouresmali, & Sadeghi Mowlaie, 2016; Hampstead, Briceno, Mascaro, Mourdoukoutas, & Bikson, 2016). The study results showed that tDCS effectively reduces the anxiety of school-aged children who stutter, shown in Spence. No research has been done on the effect of tDCS on reducing anxiety in school-aged children who stutter. Since stuttering is closely related to anxiety, this study shows that tDCS reduces anxiety in these people.
Taherifard, Saeidmanesh, and Azizi (2020), after a systematic review on the effectiveness of tDCS on anxiety, argued that anxiety in stammering adolescents affected by tDCS was reduced significantly. So, it seems that tDCS on both sides of the anterior temporal region can effectively treat the anxiety of stuttering. The tDCS over the left IFC during the fluent mode of speaking facilitated plasticity of the frontal speech network and prolonged its normalized functioning, resulting in lasting improvements in fluency. As expected, tDCS enhanced fluency in children who stutter and reduced anxiety compared with the sham group.
5. Conclusion
In general, we can say that tDCS is an appropriate intervention for improving the severity of the stuttering of school-aged children and can be considered an effective intervention on their anxiety. In conclusion, the present tDCS study demonstrated that speech fluency was significantly improved by the tDCS montage of an anodal electrode over the left inferior frontal cortex in children who stutter.
The limitation of the present study is its implementation on only two clinics in Tehran and on school-aged children. Therefore, it is suggested that this research be done in other cities. Additionally, in this study, the subjects included students aged 8 to 12 years. Further studies are required to support our presumptions regarding age influences (on adults with stuttering). Moreover, we examined the effect of tDCS treatment on reducing stuttering symptoms. So, it is also suggested to evaluate the effectiveness of this method compared to other methods.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. All study participants completed the treatment consent form with full knowledge and consent. The subject, purpose, and procedure of the study were explained to all participants. Furthermore, the research participants were allowed to leave the study whenever desired.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
References
Alizadeh Goradel, J, Pouresmali, A, & Sadeghi Mowlaie, M. (2016). The effects of transcranial direct current stimulation on obsession-compulsion, anxiety, and depression of a patient suffering from obsessive-compulsive disorder. Journal of Practice Clinical Psychology, 4(2), 75-80. [DOI:10.15412/J.JPCP.06040201]
Allman, C., Amadi, U., Winkler, A. M., Wilkins, L., Filippini, N., & Kischka, U., et al. (2016). Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Science Translation Medicine, 8(330), 330re1. [DOI:10.1126/scitranslmed.aad5651] [PMID] [PMCID]
Baker, J. M., Rorden, C., & Fridriksson, J. (2010). Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke, 41(6), 1229-36. [DOI:10.1161/STROKEAHA.109.576785] [PMID] [PMCID]
Belyk, M., Kraft, S. J., & Brown, S. (2015). Stuttering as a trait or state meta-analysis of neuroimaging studies. Journal of Neuroscience, 41(2), 275-84, [DOI:10.1111/ejn.12765] [PMID]
Bestmann, S., de Berker, A. O., & Bonaiuto, J. (2015). Understanding the behavioural consequences of noninvasive brain stimulation. Trends in Cognitive Sciences, 19(1), 13-20. [DOI:10.1016/j.tics.2014.10.003] [PMID]
Bindman, L. J., Lippold, O. C., & Redfearn, J. W. (1964). The action of brief polarizing currents on the cerebral cortex of the rat 1 during current flow and 2 in the production of long-lasting after-effects. Journal of Physiology, 172(3), 369-82. [DOI:10.1113/jphysiol.1964.sp007425] [PMID] [PMCID]
Blood, G. W., & Blood, I. M. (2007). Preliminary study of self-reported experience of physical aggression and bullying of boys who stutter: Relation to increased anxiety. Perceptual and Motor Skills, 104(3 Pt 2), 1060-6. [DOI:10.2466/pms.104.4.1060-1066] [PMID]
Bohland, J. W., Bullock, D., & Guenther, F. H. (2010). Neural representations and mechanisms for the performance of simple speech sequences. Journal of Cognitive Neuroscience, 22(7), 1504-29. [DOI:10.1162/jocn.2009.21306] [PMID] [PMCID]
Brignell, A., Krahe, M., Downes, M., Kefalianos, E., Reilly, S., & Morgan, A. T. (2020). Asystematic review of interventions for adults who stutter. Journal of Fluency Disorder, 64, 105766. [DOI:10.1016/j.jfludis.2020.105766] [PMID]
Brown, S., Ingham, R. J., Ingham, J. C., Laird, A. R., & Fox, P. T. (2005). Stuttered and fluent speech production: An ALE meta-analysis of functional neuroimaging studies. Human Brain Mapping, 25(1), 105-17. DOI:10.1002/hbm.20140] [PMID] [PMCID]
Budde, K. S., Barron, D. S., & Fox, P. T. (2014). Stuttering, induced fluency, and natural fluency: A hierarchical series of activation likelihood estimation meta-analyses. Brain Language, 139, 99-107. [DOI:10.1016/j.bandl.2014.10.002] [PMID] [PMCID]
Caruso, A. J., Chodzko-Zajko, W. J., Bidinger, D. A., & Sommers, R. K. (1994). Adults who stutter: Responses to cognitive stress. Journal of Speech and Hearing Research, 37(4), 746-54. [DOI:10.1044/jshr.3704.746] [PMID]
Chang, S. E., Angstadt, M. Chow, H. M., Etchell, A. C., Garnett, E. O., & Choo, A. L., et al. (2018). Anomalus nework architecture of the resting brain in children who stutter. Journal of Fluency Disorder, 55, 46-67. [DOI:10.1016/j.jfludis.2017.01.002] [PMID] [PMCID]
Chesters, J., Watkins, K. E., Mottonen, R. (2017). Investigating the feasibility of using transcranial direct current stimulation to enhance fluency in people who stutter. Journal of Brain & Language, 164, 68-76. [DOI:10.1016/j.bandl.2016.10.003] [PMID] [PMCID]
Chesters, J., Mottonen, R., & Watkins, K. E. (2018). Transcranial direct current stimulation over left inferior frontal cortex improves speech fluency in adults who stutter. Brain, 141(4), 1161-71. [DOI:10.1093/brain/awy011] [PMID] [PMCID]
Craig, A., Blumgart, E., & Tran, Y. (2009). The impact of stuttering on the quality of life in adults who stutter. Journal of Fluency Disorder, 34(2), 61-71. [DOI:10.1016/j.jfludis.2009.05.002] [PMID]
Elsberg, C. A. (1917). Experiments on motor nerve regeneration and the direct neurotization of paralyzed muscles by their own and by foreign nerves. Science, 45(1161), 318-20. [DOI:10.1126/science.45.1161.318] [PMID]
Filmer, H. L., Dux, P. E., & Mattingley, J. B. (2014). Applications of transcranial direct current stimulation for understanding brain function. Trends of Neuroscience, 37(12), 742-53. [DOI:10.1016/j.tins.2014.08.003] [PMID]
Fiori, V., Coccia, M., Marinelli, C. V., Vecchi, P., Bonifazi, S., & Ceravolo, M. G. et al. (2011). Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of Cognitive Neuroscience, 23(9), 2309-23. [DOI:10.1162/jocn.2010.21579] [PMID]
Fitzgerald, H. E., Djurdjic, S. D., & Maguin, E. (1992). Assessment of sensitivity to interpersonal stress in stutterers. Journal of Fluency Disorders, 25(1), 31-42. [DOI:10.1016/0021-9924(92)90012-L]
Fox, P. T., Ingham, R. J., Ingham, J. C. Hirsch, T. B., Downs, J. H., & Martin, C., et al. (1996). A PET study of the neural systems of stuttering. Nature, 382(6587), 158-62. [DOI:10.1038/382158a0] [PMID]
Garnett, E. D., Chow, H. M., Choo, A. L., & Chang, S. E. (2019). Stuttering severity modulates effects of noninvasive brain stimulation in adults who stutter. Frontiers in Human Neuroscience, 13, 411. [DOI:10.3389/fnhum.2019.00411] [PMID] [PMCID]
Hampstead, B. M., Briceno, E., Mascaro, N., Mourdoukoutas, M., & Bikson, A. (2016). Current status of transcranial direct current stimulation in posttraumatic stress and other anxiety disorders. Current Behavioral Neuroscience Reports, 3(2), 95-101. [DOI:10.1007/s40473-016-0070-9] [PMID] [PMCID]
Heth, I., & Lavidor, M. (2015). Improved reading measures in adults with dyslexia following transcranial direct current stimulation treatment. Neuropsychologia, 70, 107-13. [DOI:10.1016/j.neuropsychologia.2015.02.022] [PMID]
Hern, J. E., Landgren, S., Phillips, C. G., & Porter, R. (1962). Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon’s motor cortex. Journal of Physiology, 161(1), 73-90. [DOI:10.1113/jphysiol.1962.sp006874] [PMID] [PMCID]
Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393-402. [DOI:10.1038/nrn2113] [PMID]
Holland, R., & Crinion, J. (2012). Can tDCS enhance treatment of aphasia after stroke? Aphasiology, 26(9), 1169-91. [DOI:10.1080/02687038.2011.616925] [PMID] [PMCID]
Iverach, L., O’Brian, S., Jones, M., Block, S., Lincoln, M., & Harrison, E., et al. (2009). Prevalence of anxiety disorders among adults seeking speech therapy for stuttering. Journal of Anxiety Disorder, 23(7), 928-34. [DOI:10.1016/j.janxdis.2009.06.003] [PMID]
Jacobson, L., Koslowsky, M., & Lavidor, M. (2012). tDCS polarity in motor and cognitive domains: A meta-analytical review. Experimental Brain Research, 216(1), 1-10.[DOI:10.1007/s00221-011-2891-9] [PMID]
Klein, J. F., & Hood, S. B. (2004). The impact of stuttering on employment opportunities and job performance. Journal of Fluency Disorder, 29(4), 255-73. [DOI:10.1016/j.jfludis.2004.08.001] [PMID]
Khedr, E. M., Shawky, O. A., El-Hammady, D. H., Rothwell, J. C., Darwish, E. S., & Mostafa, O. M., et al. (2013). Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: A pilot randomized controlled trial. Neurorehabilitation Neural Repair, 27(7), 592-601. [DOI:10.1177/1545968313484808] [PMID]
Kraaimaat, F. W., Vanryckeghem, M., & Van Dam-Baggen, R. (2002). Stuttering and social anxiety. Journal of Fluency Disorders, 27(4), 319-31. [DOI:10.1016/S0094-730X(02)00160-2]
Liebetanz, D., Nitsche, M. A., Tergau, F., & Paulus, W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain, 125(Pt 10), 2238-47. [DOI:10.1093/brain/awf238] [PMID]
Marangolo, P., Fiori, V., Calpagnano, M. A., Campana, S., Razzano, C., & Caltagirone, C., et al. (2013). tDCS over the left inferior frontal cortex improves speech production in aphasia. Frontiers of Human Neuroscience, 7, 539. [DOI:10.3389/fnhum.2013.00539][PMID][PMCID]
Marangolo, P., Marinelli, C. V., Bonifazi, S., Fiori, V., Ceravolo, M. G., & Provinciali, L., et al. (2011). Electrical stimulation over the left Inferior Frontal Gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behavior Brain Research, 225(2), 498-504. [DOI:10.1016/j.bbr.2011.08.008] [PMID]
McAlister, J. (2016). Behavioral, emotional and social development of children who stutter. Journal of Fluency Disorders, 50, 23-32. [DOI:10.1016/j.jfludis.2016.09.003] [PMID]
Mortensen, J., Figlewski, K., & Andersen, H. (2016). Combined transcranial direct current stimulation and home-based occupational therapy for upper limb motor impairment following intracerebral hemorrhage: A double-blind randomized controlled trial. Disability and Rehabilitation, 38(7), 637-43. [DOI:10.3109/09638288.2015.1055379] [PMID]
Monti, A., Ferrucci, R., Fumagalli, M., Mameli, F., Cogiamanian, F., & Ardolino, G., et al. (2013). Transcranial direct current stimulation (tDCS) and language. Journal of Neurology, Neurosurgery & Psychiatry, 84(8), 832-42. [DOI:10.1136/jnnp-2012-302825] [PMID] [PMCID]
Mousavi, R., Moradi, A. R., Farzad, V., Mahdavi Harsini, S. S., Spence, & Navabinejad, S. (2007). Psychometric properties of the spence children’s anxiety scale with an Iranian sample. International Journal of Psychology, 1(1), 17-26. http://www.ijpb.ir/article_55486.html
Neumann, K., Preibisch, C., Euler, H. A., von Gudenberg, A. W., Lanfermann, H., & Gall, V., et al. (2005). Cortical plasticity associated with stuttering therapy. Journal of Fluency Disorder, 30(1), 23-39. [DOI:10.1016/j.jfludis.2004.12.002] [PMID]
Nitsche, M. A., Fricke, K., Henschke, U., Schlitterlau, A., Liebetanz, D., & Lang, N., et al. (2003). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. Journal of Physiology, 553(Pt 1), 293-301. [DOI:10.1113/jphysiol.2003.049916] [PMID] [PMCID]
Nitsche, M. A., & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology, 527(3), 633-9. [DOI:10.1111/j.1469-7793.2000.t01-1-00633.x] [PMID] [PMCID]
Nitsche, M. A., & Paulus, W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10), 1899-901. [DOI:10.1212/WNL.57.10.1899] [PMID]
Nitsche, M. A., Nitsche, M. S., Klein, C. C., Tergau, F., Rothwell, J. C., & Paulus, W. (2003). Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clinical Neurophysiology, 114(4), 600-4. [DOI:10.1016/S1388-2457(02)00412-1]
Reis, J., Schambra, H. M., Cohen, L. G., Buch, E. R., Fritsch, B., & Zarahn, E., et al. (2009). Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Science USA, 106(5), 1590-5. [DOI:10.1073/pnas.0805413106] [PMID] [PMCID]
Riley, G. D. (2009). Stuttering severity instrument: SSI4. Austin: Pro-Ed. https://books.google.com/books?id=cOnmmgEACAAJ&dq
Saltuklaroglu, T., Kalinowski, J., Robbins, M., Crawcour, S., & Bowers, A. (2009). Comparisons of stuttering frequency during and after speech initiation in unaltered feedback, altered auditory feedback and choral speech conditions. International Journal of Language & Communication Disorders, 44(6), 1000-17. [DOI:10.1080/13682820802546951] [PMID]
Sandars, M., Cloutman, L., & Woollams, A. M. (2016). Taking sides: An integrative review of the impact of laterality and polarity on efficacy of therapeutic transcranial direct current stimulation for anomia in chronic poststroke aphasia. Neural Plasticity, 2016, 8428256. [DOI:10.1155/2016/8428256] [PMID] [PMCID]
Schneier, F. R., Wexler, K. B., & Liebowitz, M. R. (1997). Social phobia and stuttering. The American Journal of Psychiatry, 154(1), 131. [DOI:10.1176/ajp.154.1.131] [PMID]
Bazargan, A., Sarmad, Z., Hejazi, E. (2007). Research methods in behavioral sciences, Tehran: Agah Publication, 11th edition. https://www.gisoom.com/bookC/
Stagg, C. J., Best, J. G., Stephenson, M. C., O’Shea, J., Wylezinska, M., & Kincses, Z. T., et al. (2009). Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. Journal of Neuroscience, 29(16), 5202-6. [DOI:10.1523/JNEUROSCI.4432-08.2009] [PMID] [PMCID]
Taherifard, M., Saeidmanesh, M., Azizi, M. (2020). The effectiveness of transcranial direct current stimulation on the anxiety and severity of stuttering in adolescents aged 15 to 18. Journal of Research on Rehabilitation Sciences, 16, 224-31. [DOI:10.22122/jrrs.v16.3605]
Toyomura, A., Fujii, T., & Kuriki, S. (2011). Effect of external auditory pacing on the neural activity of stuttering speakers. Neuroimage, 57(4), 1507-16. [DOI:10.1016/j.neuroimage.2011.05.039] [PMID]
Watkins, K. E., Smith, S. M., Davis, S., & Howell, P. (2008). Structural and functional abnormalities of the motor system in developmental stuttering. Brain, 131(Pt 1), 50-9. [DOI:10.1093/brain/awm241] [PMID] [PMCID]
Wu, J. C., Maguire, G., Riley, G., Fallon, J., LaCasse, L., & Chin, S., et al. (1995). A positron emission tomography [18F] deoxyglucose study of developmental stuttering Neuroreport. Journal of Rapid Communication of Research in Neuroscience, 6(3), 501-5. [DOI:10.1097/00001756-199502000-00024] [PMID]
Wiethoff, S., Hamada, M., & Rothwell, J. C. (2014). Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimulation, 7(3), 468-75. [DOI:10.1016/j.brs.2014.02.003] [PMID]
Developmental stuttering is a neurodevelopmental condition disrupting the smooth flow of speech, resulting in characteristic speech disfluencies. Developmental stuttering has been associated with reduced educational and employment opportunities (Klein & Hood , 2004), social anxiety (Iverach et al., 2009), and compromised quality of life (Craig, Blumgart, & Tran, 2009). The fluency of speech is interrupted by moments of stuttering, which include repetitions and prolongation of speech sounds and ‘blocks’ during which speech sounds cannot be produced (Chesters, Mottonen, & Watkins, Smith, Davis, & Howell, 2018).
Stuttering is accompanied by numerous negative consequences across the lifespan, which may increase vulnerability to social and psychological challenges (Schneier, Wexler, & Liebowitz, 1997). These negative consequences of stuttering result in the development of anxiety (Blood & Blood, 2007). Empirical studies of anxiety and stuttering have mostly supported a positive relationship between them (Kraaimaat, Vanryckeghem, & Van Dam-Baggen, 2002). In addition, a positive relationship between the severity of stuttering and the level of anxiety has been reported (Caruso, Chodzko-Zajko, Bidinger, & Sommers, 1994; Fitzgerald, Djurdjic, & Maguin, 1992). Children who stutter may begin to show impaired behavioral, emotional, and social development as early as age 3, and these difficulties are established in older children who stutter (McAlister, 2016).
People Who Stutter (PWS) show subtle abnormalities in the structure and function of the brain regions supporting speech. In particular, the Inferior Frontal Cortex (IFC) is consistently highlighted as the affected region. The IFC plays a crucial role in speech production, comprising parts involved in motor planning and integration of sensory signals (Bohland, Bullock, & Guenther, 2010; Hickok & Poeppel, 2007). Watkins et al. showed that a portion of left IFC – the ventral premotor cortex – was under-active during speaking and that the white matter underlying this region was disrupted (Watkins et al., 2008).
Scientists have been studying the effect of direct current stimulation since the early 1900s (Bindman, Lippold, & Redfearn,1964; Elsberg 1917; Fuortes 1954; Hern, J. E., Landgren, Phillips, & Porter, 1962). Nevertheless, this research technique was ignored for all intents and purposes for several decades. In the late 1990s, an interest in the transcranial Direct Current Stimulation (tDCS) effect on the human central nervous system re-emerged (Jacobson, Koslowsky, & Lavidor, 2012). Stuttering status and persistence are associated with aberrant network connectivity involving the default mode network and its connections with attention, somatomotor, and frontoparietal networks (Chang et al., 2018).
tDCS is a noninvasive brain stimulation method, which could improve the outcomes of fluency interventions in people who stutter (Chesters, Watkins, & Mottonen, 2017). tDCS modulates cortical excitability by applying weak electrical currents in the form of Direct Current (DC) brain polarization. Depending on direct current polarity, neuronal firing rates increase or decrease, presumably due to DC-induced changes in resting membrane potentials (Liebetanz, Nitsche, Tergau, & Paulus, 2002; Nitsche et al., 2003b), with anodal tDCS in most settings increasing, and cathodal tDCS decreasing motor-cortical excitability (Nitsche & Paulus, 2000, 2001).
In tDCS, neuroplastic effects emerge a short while after stimulation. The process depends on alterations to glutamatergic and GABAergic activities (Nitsche et al., 2003; Stagg et al., 2009). Like the acute membrane polarization effects, anodal tDCS and cathodal tDCS result in excitability-enhancing and excitability-reducing plasticity, respectively (Nitsche et al., 2003; Nitsche & Paulus, 2000).
Several functional imaging studies demonstrated reduced activity during speech production in Broca’s Area (BA) (i.e., the cortical center for speech production) (Fox et al., 1996; Neumann et al., 2005). Conversely, the right homolog region of Broca’s area showed overactivation; this finding has been highly replicated in several studies, including a recent meta-analysis (Brown, Ingham, Ingham, Laird, & Fox, 2005; Budde, Barron, & Fox, 2014; Belyk, Kraft, & Brown, 2015).
Previous studies have examined the effects of neuronal modulation by tDCS on language processing for healthy and clinical populations. For healthy volunteers, several lines of positive evidence have been reported, including facilitatory effects on language production for words and sentences and language learning. Also, 18–20 clinical tDCS studies have reported facilitation effects on impaired language functions in aphasic patients (Fiori et al., 2011; Marangolo et al., 2013). Stuttering severity significantly modulated the impact of stimulation: active stimulation attenuated the atypically strong association between stuttering severity and right thalamocortical network activity, especially in more severe speakers (Garnett, Chow, Choo and Chang, 2019).
Chesters investigated the effect of 5 sessions of anodal tDCS over the left inferior frontal gyrus during a speech fluency intervention on stuttering. Speech fluency significantly improved in the treatment group that received anodal tDCS combined with the fluency intervention compared to the respective sham tDCS group. Thus, using tDCS simultaneously with fluency training can enhance speech fluency in adults who stutter (Chesters et al., 2018).
These improvements are created and stabilized when tDCS is applied in consecutive daily sessions (Reis et al., 2009; Baker, Rorden, & Fridriksson, 2010). Increasingly, tDCS is being investigated as an adjunctive treatment for acquired disorders of motor, language, and cognitive functions (Baker et al., 2010; Marangolo et al., 2011; Khedr et al., 2013; Allman et al., 2016; Mortensen et al., 2016).
Despite advances in stuttering treatment, significant limitations such as instability of treatment outcomes and lack of long-term results have to be addressed (Chesters et al., 2017). Given the past evidence that tDCS can induce plasticity-like changes in cortical functions that outlast the stimulation period, we anticipate that tDCS will help stabilize intervention-related improvements in speech fluency (Nitsche & Paulus, 2001). Speech restructuring interventions were found to reduce stuttering in adults, however, with various degrees and maintenance of fluency. tDCS reduce stuttering frequency by 22%-27% (Brignell, et al., 2020).
This study aimed to evaluate the effect of tDCS on the severity of stuttering and anxiety in school-aged children. I expected that fluency intervention when used anodal tDCS would result in reduced disfluency (i.e., improved fluency) relative to the same fluency intervention with sham stimulation. My research is the first study to investigate tDCS on the severity of stuttering and anxiety in school-aged children.
2. Materials and Methods
This quasi-experimental study has a pretest-posttest and follow-up design with a control group. The study population was all elementary school students who stutter and live in Tehran City, Iran, in 2019-2020. Twenty-two right-handed children who were native Persian speakers (12 boys and 10 girls; aged 8-12 years; median: 10 years) participated in this study. The sample size was calculated based on formerly established studies and methods (Bazargan, Sarmad & Hejazi, 2007). Hence, based on similar research conducted in the past and considering the cost of testing, the sample was selected. The inclusion criteria were the presence of developmental stuttering and a participant age between 8 and 12 years. The exclusion criteria were any speech and language disorder other than developmental stuttering, personal history of seizures, diagnosis of autism spectrum disorder or attention deficit hyperactivity disorder, and neurological or psychiatric illnesses. These criteria were evaluated by a psychiatrist based on DSM-5. In this study, I delivered 1 mA of anodal tDCS over the left IFC for 20 min per day in 15 consecutive daily sessions. Fluency was assessed 1 and 6 weeks after the 15-day intervention. tDCS was performed by the author of the article, who had a tDCS certificate.
Twenty-two participants were assigned in two groups of experimental and sham-controlled. Before the study, the stuttering in the study children was confirmed by a speech therapist. Thus, every participant met the diagnostic criteria of stuttering in the DSM-5. The Stuttering Severity Instrument, version 4 (SSI-4: Riley 2009), was used as a standardized measure of stuttering symptoms, and the Spence scale was used to assess the anxiety severity. All children were evaluated by SSI-4 and Spence scale (by a speech therapist). All parents of participants in this study complete the treatment consent form with full knowledge and consent. Throughout the study, all the ethical guidelines were observed. The subject, purpose, and procedure of the study were explained to all participants. Furthermore, the research participants were allowed to leave the study whenever desired.
Eleven children in the experimental group underwent 15 sessions (5 sessions per week, based on research done in the past) of anodal tDCS for the left IFC. In the experimental group, participants received 20 min of stimulation at 1 mA using 5*7 cm electrodes during the fluency intervention. The anode was placed over the left inferior frontal cortex (centered on F7 according to the 10-20 EEG electrode placement system) and cathode over the right supra-orbital ridge. This montage was tested in a previous study of speech facilitation (Chesters et al., 2017; (Holland, & Crinion, 2012). Electrode position F7 is centered on Broca’s region, extending posteriorly to cover ventral portions of the premotor and primary motor cortex, where the representation of the articulators is located (Bestmann, de Berker, & Bonaiuto, (2015). Similar electrode placement was used in the sham-controlled group, during which the current was ramped up over 15 s, maintained for 15 s at 1 mA, and ramped down over 15 s at the start of the session. For sham stimulation, I delivered a small current pulse every 55 s throughout 20 min. These sham stimulation parameters were delivered at an ineffective current dosage. The researcher of this study performed this intervention.
Stuttering Severity Instrument, version 4 (SSI-4)
To calculate the stuttering frequency, I used SSI-4 (Riley, 2009), which provides a standardized and norm-referenced index of disfluency. This inventory assesses three domains of stuttering: frequency, duration, and physical concomitants. Speech disfluency was measured by the speech therapist at baseline, before and after the intervention, and at 1 week and 6 weeks post-intervention. The reliability of this test was obtained using the Cronbach α for the whole test as 0.93 (Riley, 2009). Garmatani, Shafiei, Feizi, Salehi, & Howell, (2012) determined the Cronbach coefficient of the whole Persian version of this tool as 0.98.
Spence Children Anxiety Scale (SCAS)
As there is an increased prevalence of anxiety in developmental stuttering (Iverach et al., 2009, 2011), all children completed the Spence Children Anxiety Scale (SCAS; Spence, 1998, quoted in Mousavi, 2007). I wanted to determine whether the tDCS could decrease the anxiety symptoms. The Spence children anxiety scale was developed to assess the severity of anxiety symptoms broadly in line with the dimensions of anxiety disorder proposed by the Diagnostic and statistical manual of mental disorders (DSM-IV). This measure consists of 44 items, of which 38 reflect specific symptoms of anxiety and 6 are positive, filler items to reduce negative response bias. This scale assesses six domains of anxiety: generalized anxiety, panic/agoraphobia, social phobia, separation anxiety, obsessive-compulsive disorder, and physical injury fears. Children are asked to rate the frequency they experience each symptom on a 4-point scale from never=0 to always=3. Anxiety was measured by a speech therapist at baseline, before and after the intervention, and at 1 week and 6 weeks post-intervention. The Cronbach α coefficient was estimated to be 0.92 for the whole instrument and between 0.60 and 0.82 for its subscales (Spence, 1998, quoted in Mousavi, 2007) examined the reliability of the Persian version of this questionnaire. The Cronbach α coefficient was found between 0.62 and 0.89, and six factors were confirmed by confirmatory factor analysis.
In this research, SPSS version 16 was used for data analysis. To analyze the effects of tDCS during the 15-day intervention on speech fluency and anxiety, we entered the change from baseline severity of stuttering and anxiety measured post-intervention into a mixed-model ANOVA. The effect of tDCS on the outcome measure (change from baseline in the SSI-4 score) was assessed using a mixed-model ANOVA with the between-subject factor of group (tDCS, sham) and a within-subjects factor of post-intervention time (baseline, 1 week, 6 weeks). The other outcome measure (change from baseline in Spens) was also assessed using a mixed-model ANOVA with the between-subject factor of group (tDCS, sham) and a within-subjects factor of post-intervention time (baseline, 1 week, 6 weeks). The means of changes from baseline in percentage of disfluent syllables, with 95% Confidence Intervals (CI), were calculated for the tDCS and sham groups separately, along with the differences in these means between the two groups. Cohen’s d was calculated for the effect sizes of the group differences. The author of this article did tDCS, and she was certified to do so.
3. Results
All participants completed the SSI-4 and Spence scale before and after the intervention and were included in all the analyses. The 1-week post-intervention session was carried out about 8 days after intervention (range 6–13 days) and the 6-week session at about 40 days after intervention (range 32–53 days). Table 1 presents the characteristics of study groups before the intervention, which were well matched between the tDCS and sham groups.

As shown in Table 1, in all test subscales in the experimental group, mean scores of stuttering severity in children in the posttest and follow-up (1-week and 6-week) decreased but in the sham group, no such changes were observed. Also, the mean score of anxiety in children in the posttest and follow-up decreased, but in the sham group, no such change was observed. The significance of group differences according to the pretest, posttest, and follow-up (1 and 6 weeks) scores was examined by mixed-model ANOVA.
Table 2 presents the results of the analysis of variance of the main effects and their interactions.

.PNG)
.PNG)
As seen in Table 2, analysis of variance result was significant for intragroup (time) and between-group of total SSI-4 factor. The interaction of group effect and time was also significant (F=451.16, df=2). The impact of the intervention in relation to group interaction and time was 0.84, and its statistical power was 1. So, tDCS was effective on the severity of stuttering.
According to Table 2, the analysis of variance result was significant for intragroup and between-group of frequency factor. The interaction of group effect and time was also significant (F=131.47, df=2). The impact of experimental action in relation to group interaction and time was 0.86, and its statistical power was 1. So, tDCS was effective on the frequency factor.
Based on Table 2, the analysis of variance result was significant for intragroup and between-group of duration factor. The interaction of group effect and time was also significant (F=189.31, df=2). The impact of the intervention in relation to group interaction and time was 0.83, and its statistical power was 1. So, tDCS was effective on the duration factor.
Table 2 indicates that the analysis of variance result was significant for intragroup (time) of physical concomitants factor and not significant for between-group factor. These results mean that regardless of the group effect, the effect of time was significant. Also, the interaction of group effect and time was significant (F=144.57, df=2). The effect of the intervention in relation to group interaction and time was 0.84, and its statistical power was 1. So, tDCS was effective on the physical concomitants factor.
Table 2 shows that the analysis of variance result was significant for intragroup and between-group of Spence total factor. The interaction of group effect and time was also significant (F=136.13, df=2). The effect of experimental action in relation to group interaction and time was 0.84, and its statistical power was 1. So, tDCS was effective on the anxiety of children who stutter.
4. Discussion
The present study aimed to examine the effectiveness of tDCS on the severity of stuttering and anxiety of children who stutter. We hypothesized that administration of tDCS to the left IFC could decrease the severity of stuttering and anxiety. Speech fluency and anxiety improved in the experimental group for at least 6 weeks after the intervention. Left IFC was targeted as a critical brain region in speech production with anodal tDCS expected to enhance neuronal excitability. This region was selected for stimulation because it has a crucial role in coordinating the planning and execution of speech movements and is typically less active in PWS than fluent speakers during speech (Chesters et al., 2017).
Our results were consistent with Marangolo et al., (2011) and Allman et al., 2016) results on the effectiveness of tDCS on reducing the severity of stuttering. Their results reflect that the tDCS parameters effectively modulate speech and motor learning (Allman et al., 2016; Marangolo et al., 2011). However, a systematic direct comparison of various tDCS protocols on speech fluency would help gain more information on the potential clinical benefits of this approach (Allman et al., 2016).
Our results are consistent with previous work in aphasia (Holland, & Crinion, 2012; Monti et al., 2013; Sandars, Cloutman, & Woollams, 2016). tDCS could be combined with different behavioral methods for increasing fluency. Previous studies chose tDCS, as they aimed to reinforce fluent speech, and choral speech induces fluency immediately and more successfully and to a greater extent than other methods (Saltuklaroglu, Kalinowski, Robbins, Crawcour, & Bowers, 2009). Temporary fluency enhancements are associated with normalized activity in the left inferior frontal cortex (Wu et al., 1995; Fox et al., 1996; Toyomura, Fujii, & Kuriki, 2011). Of particular relevance to the current trial are two studies showing increased speech motor skill following anodal tDCS over the left IFC (Marangolo et al., 2011, 2013) in two small samples of patients with acquired apraxia of speech (three and eight patients, respectively). The larger sample size of stuttering participants supports the claim that applying anodal tDCS over the left IFC can increase speech motor rehabilitation outcomes (Chesters et al., 2018).
A previous tDCS study of stuttering has also shown that the speech improvement induced by 1 mA of anodal tDCS over the left IFC was maintained for up to 6 weeks after the intervention. Although one study did not report significant speech improvement for the Broca area block in the anodal session, the effect of tDCS may be different between online and offline periods given several neuromodulatory factors induced by stimulation with various time scales (Filmer, Dux, & Mattingley, 2014).
One study showed that anodal TDCS to the primary motor cortex produces facilitation of corticospinal excitability in only approximately three-quarters of young and healthy participants (Wiethoff, Hamada, & Rothwell, 2014). Another study found that tDCS over area V5/MT combined with a 5-day course of reading therapy improved reading speed and fluency in adults with developmental dyslexia, with benefits persisting 1 week after the intervention (Heth, & Lavidor, 2015).
The SSI-4 was included in the trial as a widely recognized standardized clinical measure, which provided complementary information to the primary outcome measure regarding fluency disruptions. Specifically, the percentage of disfluent syllables is a highly sensitive measure of stuttering frequency, whereas the SSI-4 sacrifices some sensitivity (by conversion to scaled scores) but incorporates essential information regarding the duration of stuttered moments and concomitant features, such as tic-like facial or body movements (Chesters et al., 2018). The subscales of the SSI-4 were statistically analyzed separately. However, an inspection of the means showed that reductions were larger in the tDCS group than the sham group for all subscales (frequency, physical concomitants, and duration), except for the physical concomitants subscale, there were few differences between the two groups.
Much research has been done on the effect of tDCS on anxiety, and research has shown that tDCS has been effective in reducing anxiety (Alizadeh Goradel, Pouresmali, & Sadeghi Mowlaie, 2016; Hampstead, Briceno, Mascaro, Mourdoukoutas, & Bikson, 2016). The study results showed that tDCS effectively reduces the anxiety of school-aged children who stutter, shown in Spence. No research has been done on the effect of tDCS on reducing anxiety in school-aged children who stutter. Since stuttering is closely related to anxiety, this study shows that tDCS reduces anxiety in these people.
Taherifard, Saeidmanesh, and Azizi (2020), after a systematic review on the effectiveness of tDCS on anxiety, argued that anxiety in stammering adolescents affected by tDCS was reduced significantly. So, it seems that tDCS on both sides of the anterior temporal region can effectively treat the anxiety of stuttering. The tDCS over the left IFC during the fluent mode of speaking facilitated plasticity of the frontal speech network and prolonged its normalized functioning, resulting in lasting improvements in fluency. As expected, tDCS enhanced fluency in children who stutter and reduced anxiety compared with the sham group.
5. Conclusion
In general, we can say that tDCS is an appropriate intervention for improving the severity of the stuttering of school-aged children and can be considered an effective intervention on their anxiety. In conclusion, the present tDCS study demonstrated that speech fluency was significantly improved by the tDCS montage of an anodal electrode over the left inferior frontal cortex in children who stutter.
The limitation of the present study is its implementation on only two clinics in Tehran and on school-aged children. Therefore, it is suggested that this research be done in other cities. Additionally, in this study, the subjects included students aged 8 to 12 years. Further studies are required to support our presumptions regarding age influences (on adults with stuttering). Moreover, we examined the effect of tDCS treatment on reducing stuttering symptoms. So, it is also suggested to evaluate the effectiveness of this method compared to other methods.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. All study participants completed the treatment consent form with full knowledge and consent. The subject, purpose, and procedure of the study were explained to all participants. Furthermore, the research participants were allowed to leave the study whenever desired.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
References
Alizadeh Goradel, J, Pouresmali, A, & Sadeghi Mowlaie, M. (2016). The effects of transcranial direct current stimulation on obsession-compulsion, anxiety, and depression of a patient suffering from obsessive-compulsive disorder. Journal of Practice Clinical Psychology, 4(2), 75-80. [DOI:10.15412/J.JPCP.06040201]
Allman, C., Amadi, U., Winkler, A. M., Wilkins, L., Filippini, N., & Kischka, U., et al. (2016). Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Science Translation Medicine, 8(330), 330re1. [DOI:10.1126/scitranslmed.aad5651] [PMID] [PMCID]
Baker, J. M., Rorden, C., & Fridriksson, J. (2010). Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke, 41(6), 1229-36. [DOI:10.1161/STROKEAHA.109.576785] [PMID] [PMCID]
Belyk, M., Kraft, S. J., & Brown, S. (2015). Stuttering as a trait or state meta-analysis of neuroimaging studies. Journal of Neuroscience, 41(2), 275-84, [DOI:10.1111/ejn.12765] [PMID]
Bestmann, S., de Berker, A. O., & Bonaiuto, J. (2015). Understanding the behavioural consequences of noninvasive brain stimulation. Trends in Cognitive Sciences, 19(1), 13-20. [DOI:10.1016/j.tics.2014.10.003] [PMID]
Bindman, L. J., Lippold, O. C., & Redfearn, J. W. (1964). The action of brief polarizing currents on the cerebral cortex of the rat 1 during current flow and 2 in the production of long-lasting after-effects. Journal of Physiology, 172(3), 369-82. [DOI:10.1113/jphysiol.1964.sp007425] [PMID] [PMCID]
Blood, G. W., & Blood, I. M. (2007). Preliminary study of self-reported experience of physical aggression and bullying of boys who stutter: Relation to increased anxiety. Perceptual and Motor Skills, 104(3 Pt 2), 1060-6. [DOI:10.2466/pms.104.4.1060-1066] [PMID]
Bohland, J. W., Bullock, D., & Guenther, F. H. (2010). Neural representations and mechanisms for the performance of simple speech sequences. Journal of Cognitive Neuroscience, 22(7), 1504-29. [DOI:10.1162/jocn.2009.21306] [PMID] [PMCID]
Brignell, A., Krahe, M., Downes, M., Kefalianos, E., Reilly, S., & Morgan, A. T. (2020). Asystematic review of interventions for adults who stutter. Journal of Fluency Disorder, 64, 105766. [DOI:10.1016/j.jfludis.2020.105766] [PMID]
Brown, S., Ingham, R. J., Ingham, J. C., Laird, A. R., & Fox, P. T. (2005). Stuttered and fluent speech production: An ALE meta-analysis of functional neuroimaging studies. Human Brain Mapping, 25(1), 105-17. DOI:10.1002/hbm.20140] [PMID] [PMCID]
Budde, K. S., Barron, D. S., & Fox, P. T. (2014). Stuttering, induced fluency, and natural fluency: A hierarchical series of activation likelihood estimation meta-analyses. Brain Language, 139, 99-107. [DOI:10.1016/j.bandl.2014.10.002] [PMID] [PMCID]
Caruso, A. J., Chodzko-Zajko, W. J., Bidinger, D. A., & Sommers, R. K. (1994). Adults who stutter: Responses to cognitive stress. Journal of Speech and Hearing Research, 37(4), 746-54. [DOI:10.1044/jshr.3704.746] [PMID]
Chang, S. E., Angstadt, M. Chow, H. M., Etchell, A. C., Garnett, E. O., & Choo, A. L., et al. (2018). Anomalus nework architecture of the resting brain in children who stutter. Journal of Fluency Disorder, 55, 46-67. [DOI:10.1016/j.jfludis.2017.01.002] [PMID] [PMCID]
Chesters, J., Watkins, K. E., Mottonen, R. (2017). Investigating the feasibility of using transcranial direct current stimulation to enhance fluency in people who stutter. Journal of Brain & Language, 164, 68-76. [DOI:10.1016/j.bandl.2016.10.003] [PMID] [PMCID]
Chesters, J., Mottonen, R., & Watkins, K. E. (2018). Transcranial direct current stimulation over left inferior frontal cortex improves speech fluency in adults who stutter. Brain, 141(4), 1161-71. [DOI:10.1093/brain/awy011] [PMID] [PMCID]
Craig, A., Blumgart, E., & Tran, Y. (2009). The impact of stuttering on the quality of life in adults who stutter. Journal of Fluency Disorder, 34(2), 61-71. [DOI:10.1016/j.jfludis.2009.05.002] [PMID]
Elsberg, C. A. (1917). Experiments on motor nerve regeneration and the direct neurotization of paralyzed muscles by their own and by foreign nerves. Science, 45(1161), 318-20. [DOI:10.1126/science.45.1161.318] [PMID]
Filmer, H. L., Dux, P. E., & Mattingley, J. B. (2014). Applications of transcranial direct current stimulation for understanding brain function. Trends of Neuroscience, 37(12), 742-53. [DOI:10.1016/j.tins.2014.08.003] [PMID]
Fiori, V., Coccia, M., Marinelli, C. V., Vecchi, P., Bonifazi, S., & Ceravolo, M. G. et al. (2011). Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of Cognitive Neuroscience, 23(9), 2309-23. [DOI:10.1162/jocn.2010.21579] [PMID]
Fitzgerald, H. E., Djurdjic, S. D., & Maguin, E. (1992). Assessment of sensitivity to interpersonal stress in stutterers. Journal of Fluency Disorders, 25(1), 31-42. [DOI:10.1016/0021-9924(92)90012-L]
Fox, P. T., Ingham, R. J., Ingham, J. C. Hirsch, T. B., Downs, J. H., & Martin, C., et al. (1996). A PET study of the neural systems of stuttering. Nature, 382(6587), 158-62. [DOI:10.1038/382158a0] [PMID]
Garnett, E. D., Chow, H. M., Choo, A. L., & Chang, S. E. (2019). Stuttering severity modulates effects of noninvasive brain stimulation in adults who stutter. Frontiers in Human Neuroscience, 13, 411. [DOI:10.3389/fnhum.2019.00411] [PMID] [PMCID]
Hampstead, B. M., Briceno, E., Mascaro, N., Mourdoukoutas, M., & Bikson, A. (2016). Current status of transcranial direct current stimulation in posttraumatic stress and other anxiety disorders. Current Behavioral Neuroscience Reports, 3(2), 95-101. [DOI:10.1007/s40473-016-0070-9] [PMID] [PMCID]
Heth, I., & Lavidor, M. (2015). Improved reading measures in adults with dyslexia following transcranial direct current stimulation treatment. Neuropsychologia, 70, 107-13. [DOI:10.1016/j.neuropsychologia.2015.02.022] [PMID]
Hern, J. E., Landgren, S., Phillips, C. G., & Porter, R. (1962). Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon’s motor cortex. Journal of Physiology, 161(1), 73-90. [DOI:10.1113/jphysiol.1962.sp006874] [PMID] [PMCID]
Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393-402. [DOI:10.1038/nrn2113] [PMID]
Holland, R., & Crinion, J. (2012). Can tDCS enhance treatment of aphasia after stroke? Aphasiology, 26(9), 1169-91. [DOI:10.1080/02687038.2011.616925] [PMID] [PMCID]
Iverach, L., O’Brian, S., Jones, M., Block, S., Lincoln, M., & Harrison, E., et al. (2009). Prevalence of anxiety disorders among adults seeking speech therapy for stuttering. Journal of Anxiety Disorder, 23(7), 928-34. [DOI:10.1016/j.janxdis.2009.06.003] [PMID]
Jacobson, L., Koslowsky, M., & Lavidor, M. (2012). tDCS polarity in motor and cognitive domains: A meta-analytical review. Experimental Brain Research, 216(1), 1-10.[DOI:10.1007/s00221-011-2891-9] [PMID]
Klein, J. F., & Hood, S. B. (2004). The impact of stuttering on employment opportunities and job performance. Journal of Fluency Disorder, 29(4), 255-73. [DOI:10.1016/j.jfludis.2004.08.001] [PMID]
Khedr, E. M., Shawky, O. A., El-Hammady, D. H., Rothwell, J. C., Darwish, E. S., & Mostafa, O. M., et al. (2013). Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: A pilot randomized controlled trial. Neurorehabilitation Neural Repair, 27(7), 592-601. [DOI:10.1177/1545968313484808] [PMID]
Kraaimaat, F. W., Vanryckeghem, M., & Van Dam-Baggen, R. (2002). Stuttering and social anxiety. Journal of Fluency Disorders, 27(4), 319-31. [DOI:10.1016/S0094-730X(02)00160-2]
Liebetanz, D., Nitsche, M. A., Tergau, F., & Paulus, W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain, 125(Pt 10), 2238-47. [DOI:10.1093/brain/awf238] [PMID]
Marangolo, P., Fiori, V., Calpagnano, M. A., Campana, S., Razzano, C., & Caltagirone, C., et al. (2013). tDCS over the left inferior frontal cortex improves speech production in aphasia. Frontiers of Human Neuroscience, 7, 539. [DOI:10.3389/fnhum.2013.00539][PMID][PMCID]
Marangolo, P., Marinelli, C. V., Bonifazi, S., Fiori, V., Ceravolo, M. G., & Provinciali, L., et al. (2011). Electrical stimulation over the left Inferior Frontal Gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behavior Brain Research, 225(2), 498-504. [DOI:10.1016/j.bbr.2011.08.008] [PMID]
McAlister, J. (2016). Behavioral, emotional and social development of children who stutter. Journal of Fluency Disorders, 50, 23-32. [DOI:10.1016/j.jfludis.2016.09.003] [PMID]
Mortensen, J., Figlewski, K., & Andersen, H. (2016). Combined transcranial direct current stimulation and home-based occupational therapy for upper limb motor impairment following intracerebral hemorrhage: A double-blind randomized controlled trial. Disability and Rehabilitation, 38(7), 637-43. [DOI:10.3109/09638288.2015.1055379] [PMID]
Monti, A., Ferrucci, R., Fumagalli, M., Mameli, F., Cogiamanian, F., & Ardolino, G., et al. (2013). Transcranial direct current stimulation (tDCS) and language. Journal of Neurology, Neurosurgery & Psychiatry, 84(8), 832-42. [DOI:10.1136/jnnp-2012-302825] [PMID] [PMCID]
Mousavi, R., Moradi, A. R., Farzad, V., Mahdavi Harsini, S. S., Spence, & Navabinejad, S. (2007). Psychometric properties of the spence children’s anxiety scale with an Iranian sample. International Journal of Psychology, 1(1), 17-26. http://www.ijpb.ir/article_55486.html
Neumann, K., Preibisch, C., Euler, H. A., von Gudenberg, A. W., Lanfermann, H., & Gall, V., et al. (2005). Cortical plasticity associated with stuttering therapy. Journal of Fluency Disorder, 30(1), 23-39. [DOI:10.1016/j.jfludis.2004.12.002] [PMID]
Nitsche, M. A., Fricke, K., Henschke, U., Schlitterlau, A., Liebetanz, D., & Lang, N., et al. (2003). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. Journal of Physiology, 553(Pt 1), 293-301. [DOI:10.1113/jphysiol.2003.049916] [PMID] [PMCID]
Nitsche, M. A., & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology, 527(3), 633-9. [DOI:10.1111/j.1469-7793.2000.t01-1-00633.x] [PMID] [PMCID]
Nitsche, M. A., & Paulus, W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10), 1899-901. [DOI:10.1212/WNL.57.10.1899] [PMID]
Nitsche, M. A., Nitsche, M. S., Klein, C. C., Tergau, F., Rothwell, J. C., & Paulus, W. (2003). Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clinical Neurophysiology, 114(4), 600-4. [DOI:10.1016/S1388-2457(02)00412-1]
Reis, J., Schambra, H. M., Cohen, L. G., Buch, E. R., Fritsch, B., & Zarahn, E., et al. (2009). Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Science USA, 106(5), 1590-5. [DOI:10.1073/pnas.0805413106] [PMID] [PMCID]
Riley, G. D. (2009). Stuttering severity instrument: SSI4. Austin: Pro-Ed. https://books.google.com/books?id=cOnmmgEACAAJ&dq
Saltuklaroglu, T., Kalinowski, J., Robbins, M., Crawcour, S., & Bowers, A. (2009). Comparisons of stuttering frequency during and after speech initiation in unaltered feedback, altered auditory feedback and choral speech conditions. International Journal of Language & Communication Disorders, 44(6), 1000-17. [DOI:10.1080/13682820802546951] [PMID]
Sandars, M., Cloutman, L., & Woollams, A. M. (2016). Taking sides: An integrative review of the impact of laterality and polarity on efficacy of therapeutic transcranial direct current stimulation for anomia in chronic poststroke aphasia. Neural Plasticity, 2016, 8428256. [DOI:10.1155/2016/8428256] [PMID] [PMCID]
Schneier, F. R., Wexler, K. B., & Liebowitz, M. R. (1997). Social phobia and stuttering. The American Journal of Psychiatry, 154(1), 131. [DOI:10.1176/ajp.154.1.131] [PMID]
Bazargan, A., Sarmad, Z., Hejazi, E. (2007). Research methods in behavioral sciences, Tehran: Agah Publication, 11th edition. https://www.gisoom.com/bookC/
Stagg, C. J., Best, J. G., Stephenson, M. C., O’Shea, J., Wylezinska, M., & Kincses, Z. T., et al. (2009). Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. Journal of Neuroscience, 29(16), 5202-6. [DOI:10.1523/JNEUROSCI.4432-08.2009] [PMID] [PMCID]
Taherifard, M., Saeidmanesh, M., Azizi, M. (2020). The effectiveness of transcranial direct current stimulation on the anxiety and severity of stuttering in adolescents aged 15 to 18. Journal of Research on Rehabilitation Sciences, 16, 224-31. [DOI:10.22122/jrrs.v16.3605]
Toyomura, A., Fujii, T., & Kuriki, S. (2011). Effect of external auditory pacing on the neural activity of stuttering speakers. Neuroimage, 57(4), 1507-16. [DOI:10.1016/j.neuroimage.2011.05.039] [PMID]
Watkins, K. E., Smith, S. M., Davis, S., & Howell, P. (2008). Structural and functional abnormalities of the motor system in developmental stuttering. Brain, 131(Pt 1), 50-9. [DOI:10.1093/brain/awm241] [PMID] [PMCID]
Wu, J. C., Maguire, G., Riley, G., Fallon, J., LaCasse, L., & Chin, S., et al. (1995). A positron emission tomography [18F] deoxyglucose study of developmental stuttering Neuroreport. Journal of Rapid Communication of Research in Neuroscience, 6(3), 501-5. [DOI:10.1097/00001756-199502000-00024] [PMID]
Wiethoff, S., Hamada, M., & Rothwell, J. C. (2014). Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimulation, 7(3), 468-75. [DOI:10.1016/j.brs.2014.02.003] [PMID]
Type of Study: Original Research Article |
Subject:
Psychiatry
Received: 2021/03/8 | Accepted: 2021/07/24 | Published: 2021/07/19
Received: 2021/03/8 | Accepted: 2021/07/24 | Published: 2021/07/19
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |