Volume 12, Issue 1 (Winter 2024)
PCP 2024, 12(1): 43-52 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Esmaili H, Sotoudehasl N, Ghorbani R, Maheri M. Comparison of the effectiveness of Repetitive Transcranial Magnetic Stimulation (rTMS) and Drug Therapy on Obsessive-Compulsive Disorder (OCD). PCP 2024; 12 (1) :43-52
URL: http://jpcp.uswr.ac.ir/article-1-884-en.html
URL: http://jpcp.uswr.ac.ir/article-1-884-en.html
1- Department of Psychology, Faculty of Humanity, Semnan Branch, Islamic Azad University, Semnan, Iran.
2- Department of Psychology, Faculty of Humanity, Semnan Branch, Islamic Azad University, Semnan, Iran. ,sotodeh1 yahoo.com
3- Social Determinants of Health Research Center, Semnan University of Medical Sciences, Semnan, Iran.
4- Department of Psychiatry, Faculty of Medical Sciences, Shahid Beheshti University, Tehran, Iran.
2- Department of Psychology, Faculty of Humanity, Semnan Branch, Islamic Azad University, Semnan, Iran. ,
3- Social Determinants of Health Research Center, Semnan University of Medical Sciences, Semnan, Iran.
4- Department of Psychiatry, Faculty of Medical Sciences, Shahid Beheshti University, Tehran, Iran.
Full-Text [PDF 597 kb]
(281 Downloads)
| Abstract (HTML) (1074 Views)
Full-Text: (130 Views)
Introduction
Obsessive-compulsive disorder (OCD) is an important cause of mental health-related morbidity (Fineberg, et al., 2020). World Health Organization (WHO) has classified OCD as one of ten diseases associated with notable disability globally. Its estimated prevalence in the United States is 2. 3% for lifetime OCD and 1. 2% for 12-month criteria. On the other hand, according to a study in six European countries, the lifetime prevalence of OCD in the general population is estimated between 1 and 2%. The prevalence of OCD in Iran is 1. 8% (07% and 28% in men and women, respectively) (Mohammadi, et al.2004). OCD is often associated with severe disruption in family functioning as well as impaired peer relationships and academic performance (Brezinka, Mailänder, & Walitza, 2020). OCD is associated with poor quality of life (Grochtdreis, et al., 2023) and increased risk of mortality (Kim, et al., 2023). Chronic OCD is associated with significant mental and physical morbidity, and in severe cases, it can lead to suicidal behavior, long-term hospitalization, and residential care (Fineberg, Cinosi, Smith, Busby, Wellsted, et al., 2023).
Despite the impact OCD can have on someone’s life, only a minority of those affected seek help from a psychiatrist, with about 30 to 40% receiving assistance (Swierkosz-Lenart, et al., 2023). Research is being prioritized on developing treatments that can improve health outcomes for individuals with OCD (Kammen, et al., 2021; Wang, et al., 2023; Corlier, et al., 2023). Despite receiving adequate treatment, almost 40% of patients do not respond to either treatment. This highlights the importance of developing new treatment methods. Moreover, the use of invasive treatment methods like deep brain stimulation or surgical intervention has yielded limited success (Shivakumar, et al., 2019). Other forms of intervention, such as neuromodulation techniques, are increasingly being studied as a treatment for OCD (Kammen, et al., 2021). The use of electrical currents in the brain is a common technique in neuromodulation therapies, but this current can also regulate local and network-level activities (Borgomaneri, et al., 2021). Studies have shown that approximately two-thirds of people with OCD do not achieve a satisfactory response (Wang, et al., 2023). The current research indicates that non-invasive methods of brain stimulation may be effective in alleviating OCD symptoms (Begemann, Brand, Ćurčić-Blake, Aleman, & Sommer, 2020). Transcranial magnetic stimulation (TMS), transcranial alternating current stimulation (tACS), electroconvulsive therapy (ETC), deep brain stimulation (DBS), and focused ultrasound are examples of interventions that can modify the electrical activity of the brain. These therapies are effective in neurological disorders such as anxiety, depression, and hypertension (Borgomaneri, et al., 2021). Of these, only repetitive transcranial magnetic stimulation (rTMS) and DBS show a high level of evidence in the treatment of OCD (Wang, et al., 2023; Corlier, et al., 2023). Approximately 20 years have been spent exploring the potential of using repetitive TMS as a treatment for OCD (Fitzsimmons et al., 2022).
Currently, leading treatment options for OCD include selective serotonin reuptake inhibitors (SSRIs) and cognitive behavioral therapy (CBT) (Del Casale, et al., 2019; Uhre, et al., 2020). In meta-analysis studies, SSRIs were better tolerated. To be effective, these drugs must be used in appropriate doses and durations (Zhou, et al., 2019). Additionally, meta-analyses have recommended higher doses of SSRIs for the treatment of OCD (Thamby and Jaisoorya, 2019). To evaluate the effectiveness of these drugs and replace or enhance them, the drugs must be used for at least 12 weeks. People who do not respond to one SSRI may respond to another SSRI or enhance their treatment regimen with additional medications. Most evidence-based studies have been conducted to augment first-line medications with low-dose dopamine receptor antagonists (i.e. antipsychotics), some, but not all, have confirmed their effectiveness. Other drugs have also been studied and recommended to enhance the therapeutic effects of SSRIs, including low-dose clomipramine, glutamatergic drugs, and D-cycloserine. However, drug selection in treatment-resistant patients must be based on multiple clinical considerations, including the presence of other comorbidities (Jahanbakhsh, Majidi, & Karimi, 2023). Evidence-based treatments are currently available, mainly involving CBT with exposure and response prevention, or pharmacological treatment with relapse inhibitors. The SSRI often produces disappointing results: Approximately 40% of patients do not respond and 50% require additional treatment (Fineberg, et al., 2020). Chronic OCD is associated with significant mental and physical illness, and in severe cases, it can lead to suicidal behavior, long-term hospitalization, and residential care (Pellegrini, et al., 2021).
Developing new treatments to improve health outcomes for people with OCD is an identified research priority (Fineberg, et al., 2018). Evidence from randomized controlled trials and meta-analyses support its effectiveness and have identified the orbitofrontal cortex (OFC) and supplementary motor area (SMA) as targets promising (Lusicic, et al., 2018). Deep TMS is a form of rTMS that can theoretically regulate deeper subcortical structures. Deep TMS targeting the anterior cingulate cortex is effective for OCD in 2 sham-controlled trials and has received FDA approval (Carmi, et al., 2022; Carmi, et al., 2019). In August 2020, the UK National Institute for Health and Care Excellence (NICE) stipulated that rTMS should only be used in a research context with OCD patients (Pellegrini, et al., 2022). rTMS is also relatively expensive, requires specialized technical equipment and personnel, and cannot be performed in the patient’s home. With the above considerations in mind, the clinical effectiveness of rTMS needs to be evaluated based on its potential to provide OCD patients with a safe and lasting improvement in quality of life. This scoping review identifies what we know and address the gaps in our knowledge in this area to evaluate and provide information on the potential therapeutic effects of rTMS for OCD.
Materials and Methods
The present study was quasi-experimental with a pre-post control group design. The statistical population of the present study included all patients referred to specialized clinics in the second, third, and seventh districts of Tehran City, Iran, from July to September 2022. Participants of the study were 45 OCD patients purposively selected and divided into two experimental groups (rTMS: 15 participants, pharmacotherapy: 15 participants) and a control group with 15 participants. A table of random numbers was used to randomly assign participants into groups. A priori sample size calculations show that, based on a medium effect size (η2=0. 5), a critical p of 0.05, and a critical power level of 0.95, the required sample size is 42. Finally, with the withdrawal of some participants, the estimated sample size was 45.
Patients were eligible if they: (a) were 18 years of age or older; (b) (DSM-5) defined OCD was determined by a research psychiatrist using the Mini International Neuropsychiatric Interview version 7. 02 for DSM-5; (c) duration of symptoms >1 year (based on medical history); and (d) have the Millon clinical multiaxial inventory-III or SCL-90-R obsessive-compulsive scale (OCS), which represents OCD of at least moderate severity.
Patients were excluded if they: (a) were absent from meetings, failed to complete tasks, had unforeseen events (such as illness, death of a relative, etc.), and presented expressed refusal to continue cooperation; (b) have a clinical history of schizophrenia, psychotic symptoms, bipolar disorder, Tourette’s syndrome (not excluding tics not equivalent to Tourette’s syndrome), organic psychosis physical, psychosurgical, borderline disorder or histrionic personality type; (c) had an alcohol or substance use disorder in the past 12 months; (d) have another diagnostic and statistical manual of mental disorder, 5th edition (DSM-5) considered the primary treatment target; or (e) had moderate (20-28) and severe (29-63) depression, as determined by the Beck depression inventory at baseline.
In the research administration, we had to work together with Sajad Hospital and counseling centers (Aram and Rayeheye Omid) in District Three. The researchers worked with the center officials to get the right people for their study. Then they gave them surveys and treatment. It is important to mention that after choosing the people for the study, we explained the treatment plans and goals to them. We also told them that they could choose to participate on their own, and they were not forced to join.
In a group of repetitive magnetic stimulation of the brain from the skull, the duration of sessions followed the following parameters at a rate of three sessions per week for four weeks. The location of coil was placed in the dorsolateral prefrontal cortex (DLPFC) on the left corresponding to point F3 and on the right corresponding to point F4 and SMA area based on the international 10-20 position system for electroencephalography map (EEG). Therefore, rTMS treatment was performed with the parameters specified below regarding the variables in this study. For OCD, the SMA of 1 Hz (HZ) for 20 minutes, 1200 pulses, and areas F3 and F4 were worked with the tablet. Three minutes from zone F3 (intermittent theta bursts) and 40 seconds from zone F4 (cantinu theta burst) after the intervention, people in the control group received treatment as usual.
After the end of the intervention, re-evaluation was done with the tools used in the initial evaluation. The drug interventions during the 4 weeks of the OCD group were: Fluoxetine for the first ten days, one 10 mg capsule in the morning, then one 20 mg capsule in the morning. 2- Tab. Clomipramine 25 mg. For the first ten days, one at night, then one at noon, and one at night. 3- Tab. Clonazepam / 5mg, One Tab every night. All interventions were performed by the first author of the article. Informed consent was obtained from all individual participants included in the present study. According to the results of the Kolmogorov-Smirnov test and the threshold of significance obtained for actual OCD (P=0.145), the data of the variables at two periods are normal. According to the Levine test to verify the homogeneity of variances for OCD (P=0.574) established (P>0.05), and also based on Box’s M test, the hypothesis about the equality of the covariance matrices is approved (Box’s M=0,10, 6.70). Depending on the set of assumptions, the univariate and multivariate covariance analysis for differences between the two groups in the dependent variables. Bonferroni’s post hoc test was adopted to determine the differences between groups. It should be noted that SPSS version 26 software was used for data analysis.
The tools used in this research are as follows:
Millon Clinical Multiaxial Inventory-III (MCMI-III) (Millon, 1994).
The MCMI-III is a test with 175 questions. It is for people 18 and older who can read at an eighth-grade level. The test can be done on paper, on a computer, or online and takes about 25-30 minutes to complete. The test can be scored online, with software, by mail, or by hand. After taking the test, you can get reports that explain your results. The MCMI-III test is very helpful in understanding people’s minds and has been translated into many languages. It has been used in studies that compare different cultures, including Iran (Sharifi, Moulavi, & Namdari, 2008). Problems with understanding MCMI-III include gender, ethnicity, age, and the types of codes used. The MCMI-III test has three parts and 24 different scales. It is based on Millon’s theory of personality and is similar to the DSM-III and DSM-IV diagnostic categories. The scales measure how much people reveal about themselves, how much they want to be liked, and how much they try to make themselves look bad. The clinical scales measure different levels of personality problems, like moderate and severe. The MCMI-III was picked because it is a trustworthy and accurate way to measure personality disorders. As the manual suggests, it uses a cutoff score of 85 or higher to meet the diagnostic criteria. The Persian version of the MCMI-III test was very accurate. It was tested and proved reliable (Sharifi, et al., 2008).
Symptom Checklist-90-Revision (SCL-90-R)
SCL-90-R is a multidimensional self-report symptom inventory and its derived Iranian standard version was used in this study (Alavi, et al., 2012). The SCL-90-R has 90 questions divided into nine categories: Physical problems, obsessive thoughts, sensitivity to others, feeling sad, feeling worried, anger, fear, thinking others are out to harm you, and feeling disconnected from reality. The nine different symptoms were grouped into three big categories: The global severity index shows how serious the psychiatric problem is, the positive symptom total measures how many questions were rated as a problem, and the positive symptom distress index shows how intense the symptoms are. In this research, the Iranian version of SCL-90-R was found to be reliable with a Cronbach alpha of 0. 95 and split-half reliability of 0. 88.
Results
The mean±SD age of patients in the experimental group was 45.60±0.67 years and that of the control group was 45.46±0.67 years. According to the findings, the three groups in this research are almost homogeneous in the variables of age, education, gender, and marital status. The results of the chi-square test also show that the differences between the three groups in terms of age (P=0.861), education (P=0.685), gender (P=0.433), and marital status (P=0.738) are not significant (P>0.05).
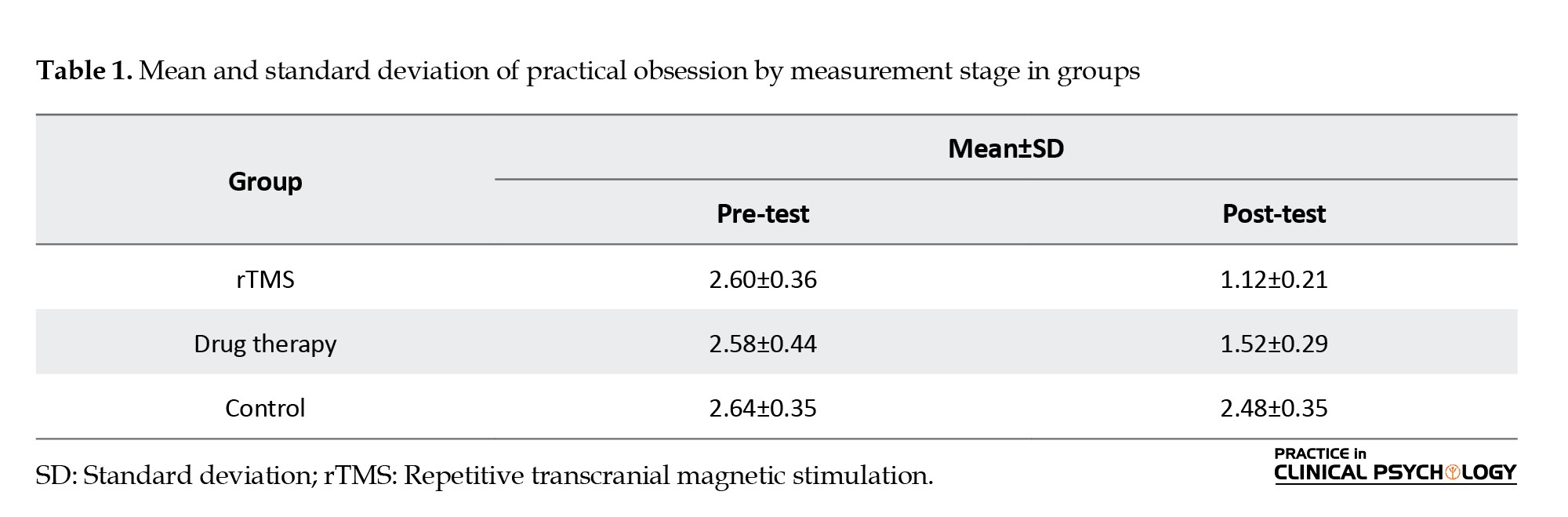
As seen, the mean in the magnetic stimulation group from the skull and the drug therapy group in the post-test stage decreases compared to the pre-test. Based on the results recorded in Table 1, we can describe that magnetic stimulation treatments from the skull and drug therapy have reduced the obsessive-compulsive components of the patients.
According to the results of significant levels obtained for OCD, the Kolmogorov Smirnov test (data normality test (P=0.145), which is greater than 0.05, and the data of the variables in both stages are normal and can be tested using parametric tests. As can be seen, the assumption of equality of variances for OCD (P=0.574) is established (P>0.05). The standard method for evaluating the equality of covariance matrices, the M box test is used where a significant level less than 0.05 is considered as an index of heterogeneity or inequality. As can be seen, the assumption of equality of covariance matrices is maintained (M box=0.10, 6.70).
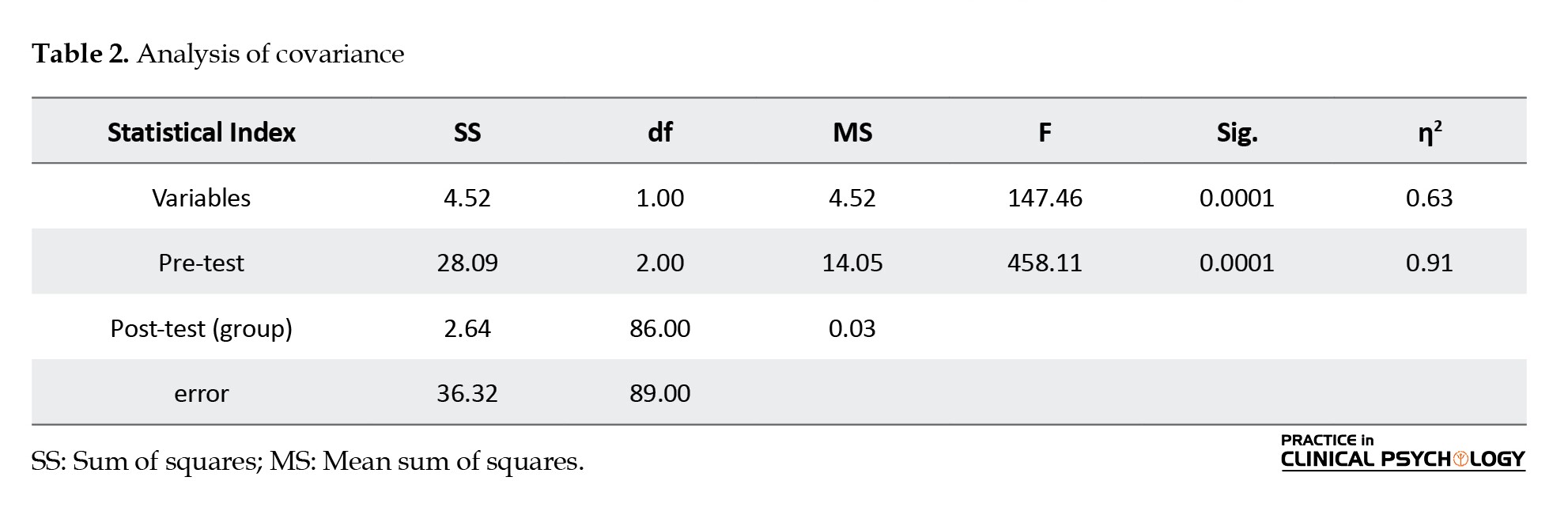
The results in Table 2 show that by removing the effects of pre-test variables and considering the calculated F-factors, there is a significant difference between the adjusted means of participants’ obsessive-compulsive scores in the post-test phase (F=458.11 P= 0.0001).
According to Table 3, the consequences of the study reasoned that the differentiation between the treatment technique of rTMS (P=0.0001) and drug administration (P=0.0001) is significant in OCD. The decrease in OCD based on rTMS was more than the drug therapy group (P=0.0017).

Discussion
The study investigated the comparison of the effectiveness of rTMS and drug administration in reducing OCD symptoms. The consequences of the study reasoned that the differentiation between the treatment technique for rTMS and drug administration is significant in OCD. The reduction in Yale-Brown obsessive–compulsive scale (YBOCS) score was significantly greater at the post-treatment assessment. In other words, in rTMS, the OCD score decreased more than in the drug treatment group. Consistent with our findings, Adu et al. (2021) found using rTMS on specific areas of the brain is both effective and well-tolerated for OCD treatment. This is especially true for the SMA and the OFC, compared to other areas like the prefrontal cortex. Although the studies varied in their results and how severe the symptoms were, rTMS seems like a good treatment for OCD. Furthermore, using low-frequency rTMS over the left dorsolateral prefrontal cortex (DLPFC) can help reduce Y-BOCS scores in OCD patients who have not responded to other treatments. This effect lasts for at least three to six months after 15 sessions of treatment. Using both antipsychotic and serotonergic medications along with rTMS therapy may help predict how well the adjunctive treatment will work (Jahanbakhsh, Majidi, & Karimi, 2023). Using high-frequency magnetic stimulation over specific parts of the brain can help improve symptoms of OCD. This could be a helpful option for people who do not see good results from medication or therapy (Carmi, et al., 2022). In recent years, several attempts to treat OCD with rTMS have been reported (Uhre, et al., 2020; Kammen, et al., 2021; Wang, et al., 2023), and a recent meta-analysis found that rTMS performed clinically and statistically superior to sham treatment (Fitzsimmons et al., 2022).
Recently, it was found that doing deep transcranial magnetic stimulation therapy for six weeks every day is safe and works well for people with OCD who do not get better with medicine or CBT (Carmi et al., 2018, 2019). Two studies recently looked at how often and where rTMS is used for OCD (Liang et al., 2021; Perera et al., 2021). They came to different conclusions about how well it works. One study found that stimulating both sides of the brain is the best treatment option (Perera et al., 2021), while another study found that stimulating the brain at a low frequency is the most effective treatment (Liang et al., 2021). This difference in interpretation may be due to the decision of the two studies not to separate and distinguish between unique frequency and location combinations. Classically, high-frequency rTMS and intermittent theta burst stimulation (iTBS) are thought to increase cortical excitability, whereas low-frequency rTMS and continuous theta burst stimulation (cTBS) are thought to causing inhibition of underlying brain tissue (Rossi et al., 2009). In the context of depression treatment, stimulation of the left or right DLPFC with high-frequency versus low-frequency rTMS may have different therapeutic effects (Brunoni et al., 2017). This indicates that frequency and location have an important and independent influence on the effectiveness of rTMS and therefore should be considered separately when evaluating and comparing different protocols.
The cause of OCD, according to brain scans, is related to a problem in a circuit of brain areas including the OFC, DLPFC, and thalamus. Changing the way the brain works with surgery and deep brain stimulation helps lessen symptoms of OCD. Considering the chance that rTMS can change parts of the brain, researchers have looked at how it could help treat OCD by targeting specific brain circuits. They have found that rTMS may help balance out abnormal brain activity in people with OCD (Adu, et al., 2021).
A lot of evidence shows that deep rTMS therapy works well for OCD, as used in the United States. The treatment will probably be used more by doctors if it is approved by the FDA. In this new report, rTMS was used because the patient had a history of reversible cerebral vasoconstriction syndrome (RCVS) with bleeding inside the head, possibly caused by fluvoxamine. The use of birth control pills at the same time might have also played a part in this. (Silveira, Damiano, Abelama Neto, Gomes, et al., 2022). In a group of 210 patients who had not started using SSRI again within 6 months to 7 years, RCVS came back in 5% of the patients in a study by Chen, et al. (2015). We do not know if starting SSRI again increases the risk of RCVS coming back. It is really important to study this and find other treatments because RCVS is very serious. In addition to causing RCVS, SSRI medicines have also been linked to a small increase in the chance of bleeding in the brain (Douros, Ades, & Renoux, 2018).
In a 2020 cohort of 1279 patients with retrocranial hemorrhage, a higher rate of recurrent intracranial hemorrhage was observed in SSRI users, and this association was greater in patients at risk of hemorrhage. Postcranial blood is higher in the ɛ4 allele, in patients with lobar intracranial hemorrhage, and patients with previous intracranial hemorrhage (Kubiszewski, et al., 2021). Researchers looked at 55 experiments testing 19 different treatments or fake treatments, with a total of 2011 people involved. Ondansetron, deep TMS, therapist-administered cognitive behavioral therapy (CBT-TA), and aripiprazole were rated as the top four treatments based on their surface under the cumulative ranking percentages. Although all four treatments had a big impact, CBT[TA] came very close to being significant but just missed it. In studies, deep TMS was found the most effective treatment for OCD in people who did not respond to serotonin reuptake inhibitor medications (Suhas, et al 2022).
Conclusion
This study revealed that rTMS is an effective neuro-stimulation therapy for OCD. Therefore, rTMS provides better results in terms of treatment effectiveness and clinical response rate. Furthermore, drug treatment appears to have a significant therapeutic effect. In subgroup analysis, it was found that DLPFC stimulation and inhibition produced better treatment effects. Larger follow-up periods and sample sizes are needed for future randomized controlled trials to study better protocols.
Limitations
There are several shortcomings of our study that need to be addressed. First, it was conducted at a tertiary care psychology center, which preferentially recruited patients with more severe levels of OCD. Second, despite calculating an adequate sample size, our sample can still be considered small, generating large confidence intervals, thus necessitating replication of our results in larger samples. Third, lack of follow-up time. Further work is needed to better evaluate the effectiveness and safety of this rTMS protocol, as well as its individualization. Therefore, rTMS may be an important alternative treatment for these individuals as well as for those with other contraindications to SSRIs.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in studies involving human participants were by the ethical standards of the institutional and or national Research Committee by the code IR.IAU.SEMNAN.REC.1402.022.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Investigation, data collection, data analysis: Hooshang Esmaili; Writing-original draft, Methodology: Hooshang Esmaili; Conceptualization and Supervision: Nemat Sotoudeh Asl; Writing–review & editing: Nemat Sotoudeh Asl and Raheb Ghornabni.
Conflict of interest
The authors declare no conflict of interest.
References
Adu, M. K., Eboreime, E., Sapara, A. O., Greenshaw, A. J., Chue, P., & Agyapong, V. I. O. (2021). The use of repetitive transcranial magnetic stimulation for treatment of obsessive-compulsive disorder: A scoping review. Mental Illness, 13(1), 1–13.[DOI:10.1108/MIJ-05-2021-0002] [PMID]
Alavi, S. S., Alaghemandan, H., Maracy, M. R., Jannatifard, F., Eslami, M., & Ferdosi, M. (2012). Impact of addiction to internet on a number of psychiatric symptoms in students of isfahan universities, iran, 2010. International Journal of Preventive Medicine, 3(2), 122–127. [PMID] [PMCID]
Begemann, M. J., Brand, B. A., Ćurčić-Blake, B., Aleman, A., & Sommer, I. E. (2020). Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: A meta-analysis. Psychological Medicine, 50(15), 2465–2486. [DOI:10.1017/S0033291720003670] [PMID]
Borgomaneri, S., Battaglia, S., Sciamanna, G., Tortora, F., & Laricchiuta, D. (2021). Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neuroscience and Biobehavioral Reviews, 127, 334–352. [DOI:10.1016/j.neubiorev.2021.04.036] [PMID]
Brezinka, V., Mailänder, V., & Walitza, S. (2020). Obsessive compulsive disorder in very young children - a case series from a specialized outpatient clinic. BMC Psychiatry, 20(1), 366. [DOI:10.1186/s12888-020-02780-0] [PMID]
Brunoni, A. R., Chaimani, A., Moffa, A. H., Razza, L. B., Gattaz, W. F., & Daskalakis, Z. J., et al. (2017). Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: A systematic review with network meta-analysis. JAMA Psychiatry, 74(2), 143–152. [DOI:10.1001/jamapsychiatry.2016.3644] [PMID]
Carmi, L., Tendler, A., Bystritsky, A., Hollander, E., Blumberger, D. M., Daskalakis, J., ... & Zohar, J. (2019). Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. American Journal of Psychiatry, 176(11), 931-938. [DOI:10.1176/appi.ajp.2019.18101180] [PMID]
Carmi, L., Tendler, A., Bystritsky, A., Hollander, E., Blumberger, D. M., & Daskalakis, J., et al. (2022). Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: A prospective multicenter randomized double-blind placebo-controlled trial. Focus (American Psychiatric Publishing), 20(1), 152–159. [DOI:10.1176/appi.focus.20103] [PMID]
Chen, S. P., Fuh, J. L., Lirng, J. F., Wang, Y. F., & Wang, S. J. (2015). Recurrence of reversible cerebral vasoconstriction syndrome: A long-term follow-up study. Neurology, 84(15), 1552–1558. [DOI:10.1212/WNL.0000000000001473] [PMID]
Corlier, J., Tadayonnejad, R., Wilson, A. C., Lee, J. C., Marder, K. G., & Ginder, N. D., et al. (2023). Repetitive transcranial magnetic stimulation treatment of major depressive disorder and comorbid chronic pain: Response rates and neurophysiologic biomarkers. Psychological Medicine, 53(3), 823–832. [DOI:10.1017/S0033291721002178] [PMID]
Ferracuti, S., Lamis, D. A., Rapinesi, C., Sani, G., Girardi, P., & Kotzalidis, G. D., et al. (2019). Psychopharmacological treatment of obsessive-compulsive disorder (OCD). Current Neuropharmacology, 17(8), 710–736. [DOI:10.2174/1570159X16666180813155017] [PMID]
Douros, A., Ades, M., & Renoux, C. (2018). Risk of intracranial hemorrhage associated with the use of antidepressants inhibiting serotonin reuptake: A systematic review. CNS Drugs, 32(4), 321–334. [DOI:10.1007/s40263-018-0507-7] [PMID]
Fineberg, N. A., Cinosi, E., Smith, M. V. A., Busby, A. D., Wellsted, D., & Huneke, N. T. M., et al. (2023). Feasibility, acceptability and practicality of transcranial stimulation in obsessive compulsive symptoms (FEATSOCS): A randomised controlled crossover trial. Comprehensive Psychiatry, 122, 152371. [DOI:10.1016/j.comppsych.2023.152371] [PMID]
Fineberg, N. A., Demetrovics, Z., Stein, D. J., Ioannidis, K., Potenza, M. N., & Grünblatt, E., et al. (2018). Manifesto for a European research network into Problematic Usage of the Internet. European Neuropsychopharmacology, 28(11), 1232–1246. [DOI:10.1016/j.euroneuro.2018.08.004] [PMID]
Fineberg, N. A., Hollander, E., Pallanti, S., Walitza, S., Grünblatt, E., & Dell'Osso, B. M., et al. (2020). Clinical advances in obsessive-compulsive disorder: A position statement by the International College of Obsessive-Compulsive Spectrum Disorders. International Clinical Psychopharmacology, 35(4), 173–193. [DOI:10.1097/ YIC.0000000000000314] [PMID]
Fitzsimmons, S. M. D. D., van der Werf, Y. D., van Campen, A. D., Arns, M., Sack, A. T., & Hoogendoorn, A. W., et al. (2022). Repetitive transcranial magnetic stimulation for obsessive-compulsive disorder: A systematic review and pairwise/network meta-analysis. Journal of Affective Disorders, 302, 302–312. [DOI:10.1016/j.jad.2022.01.048] [PMID]
Grochtdreis, T., König, H. H., Gallinat, J., Konnopka, A., Schulz, H., & Lambert, M., et al. (2023). Validation of the recovering quality of life (ReQoL) questionnaires for patients with anxiety, obsessive-compulsive, stress-related, somatoform and personality disorders in Germany. Journal of Psychiatric Research, 157, 202–211. [DOI:10.1016/j.jpsychires.2022.11.032] [PMID]
Jahanbakhsh, G., Alireza Haji Seyed Javadi, S., Majidi, M., Khademi, M., & Karimi, R. (2023). Effectiveness of adjunctive low-frequency repetitive transcranial magnetic stimulation therapy over the left dorsolateral prefrontal cortex in patients with obsessive-compulsive disorder refractory to medical treatment: A double-blind, randomized clinical trial. Asian Journal of Psychiatry, 80, 103384. [DOI:10.1016/j.ajp.2022.103384] [PMID]
Kammen, A., Cavaleri, J., Lam, J., Frank, A. C., Mason, X., & Choi, W., et al. (2022). Neuromodulation of OCD: A review of invasive and non-invasive methods. Frontiers in Neurology, 13, 909264. [DOI:10.3389/fneur.2022.909264] [PMID]
Kim, H., Kim, S. H., Jeong, W., Park, Y. S., Kim, J., & Park, E. C., et al. (2023). Association between obsessive-compulsive disorder and the risk of schizophrenia using the Korean National Health Insurance Service-National Sample Cohort: A retrospective cohort study. Epidemiology and Psychiatric Sciences, 32, e9. [DOI:10.1017/S2045796023000021] [PMID]
Kubiszewski, P., Sugita, L., Kourkoulis, C., DiPucchio, Z., Schwab, K., & Anderson, C. D., et al. (2020). Association of selective serotonin reuptake inhibitor use after intracerebral hemorrhage with hemorrhage recurrence and depression severity. JAMA Neurology, 78(1), 1–8. [DOI:10.1001/jamaneurol.2020.3142] [PMID]
Liang, K., Li, H., Bu, X., Li, X., Cao, L., & Liu, J., et al. (2021). Efficacy and tolerability of repetitive transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder in adults: A systematic review and network meta-analysis. Translational Psychiatry, 11(1), 332. [DOI:10.1038/s41398-021-01453-0] [PMID]
Lusicic, A., Schruers, K. R., Pallanti, S., & Castle, D. J. (2018). Transcranial magnetic stimulation in the treatment of obsessive-compulsive disorder: Current perspectives. Neuropsychiatric Disease and Treatment, 14, 1721–1736. [DOI:10.2147/NDT.S121140] [PMID]
Mohammadi, M. R., Ghanizadeh, A., Rahgozar, M., Noorbala, A. A., Davidian, H., & Afzali, H. M., et al. (2004). Prevalence of obsessive-compulsive disorder in Iran. BMC Psychiatry, 4, 2. [DOI:10.1186/1471-244X-4-2] [PMID]
Pellegrini, L., Garg, K., Enara, A., Gottlieb, D. S., Wellsted, D., & Albert, U., et al. (2022). Repetitive transcranial magnetic stimulation (r-TMS) and selective serotonin reuptake inhibitor-resistance in obsessive-compulsive disorder: A meta-analysis and clinical implications. Comprehensive Psychiatry, 118, 152339. [DOI:10.1016/j.comppsych.2022.152339] [PMID]
Pellegrini, L., Maietti, E., Rucci, P., Burato, S., Menchetti, M., & Berardi, D., et al. (2021). Suicidality in patients with obsessive-compulsive and related disorders (OCRDs): A meta-analysis. Comprehensive Psychiatry, 108, 152246. [DOI:10.1016/j.comppsych.2021.152246] [PMID]
Perera, M. P. N., Mallawaarachchi, S., Miljevic, A., Bailey, N. W., Herring, S. E., & Fitzgerald, P. B. (2021). Repetitive transcranial magnetic stimulation for obsessive-compulsive disorder: A meta-analysis of randomized, sham-controlled trials. biological psychiatry. Cognitive Neuroscience and Neuroimaging, 6(10), 947–960. [DOI:10.1016/j.bpsc.2021.03.010] [PMID]
Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A., & Safety of TMS Consensus Group (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. [DOI:10.1016/j.clinph.2009.08.016] [PMID]
Sharifi, A. A., Moulavi, H., & Namdari, K. (2008). [The validity of MCMI-III (Millon, 1994) scales (Persian)]. Knowledge & Research in Psychology, 34, 27-38. [Link]
Shivakumar, V., Dinakaran, D., Narayanaswamy, J. C., & Venkatasubramanian, G. (2019). Noninvasive brain stimulation in obsessive-compulsive disorder. Indian Journal of Psychiatry, 61(Suppl 1), S66–S76. [DOI:10.4103/psychiatry.IndianJPsychiatry_522_18] [PMID]
Silveira, J. B., Damiano, R. F., Abelama Neto, E., Gomes, R. N. D. S. M., Klein, I., & Borrione, L., et al. (2022). Double cone coil repetitive transcranial magnetic stimulation for severe obsessive-compulsive disorder after reversible cerebral vasoconstriction syndrome with intracerebral hemorrhage: A case report. Brazilian Journal of Psychiatry, 44(5), 562-564. [DOI:10.47626/1516-4446-2022-2556]
Suhas, S., Malo, P. K., Kumar, V., Issac, T. G., Chithra, N. K., & Bhaskarapillai, B., et al. (2023). Treatment strategies for serotonin reuptake inhibitor-resistant obsessive-compulsive disorder: A network meta-analysis of randomised controlled trials. The World Journal of Biological psychiatry, 24(2), 162–177. [DOI:10.1080/15622975.2022.2082525] [PMID]
Swierkosz-Lenart, K., Dos Santos, J. F. A., Elowe, J., Clair, A. H., Bally, J. F., & Riquier, F., et al. (2023). Therapies for obsessive-compulsive disorder: Current state of the art and perspectives for approaching treatment-resistant patients. Frontiers in Psychiatry, 14, 1065812. [DOI:10.3389/fpsyt.2023.1065812] [PMID]
Thamby, A., & Jaisoorya, T. S. (2019). Antipsychotic augmentation in the treatment of obsessive-compulsive disorder. Indian Journal of Psychiatry, 61(Suppl 1), S51–S57. [DOI:10.4103/psychiatry.IndianJPsychiatry_519_18] [PMID]
Uhre, C. F., Uhre, V. F., Lønfeldt, N. N., Pretzmann, L., Vangkilde, S., Plessen, K. J., ... & Pagsberg, A. K. (2020). Systematic review and meta-analysis: cognitive-behavioral therapy for obsessive-compulsive disorder in children and adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 59(1), 64-77. [DOI:10.1016/j.jaac.2019.08.480] [PMID]
Wang, J., Hua, G., Wang, S., Guo, G., Quan, D., & Yao, S., et al. (2023). Glutamatergic neurotransmission is affected by low-frequency repetitive transcranial magnetic stimulation over the supplemental motor cortex of patients with obsessive-compulsive disorder. Journal of Affective Disorders, 325, 762–769. [DOI:10.1016/j.jad.2023.01.064] [PMID]
Zhou, D. D., Zhou, X. X., Li, Y., Zhang, K. F., Lv, Z., & Chen, et al. (2019). Augmentation agents to serotonin reuptake inhibitors for treatment-resistant obsessive-compulsive disorder: A network meta-analysis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 90, 277–287. [DOI:10.1016/j.pnpbp.2018.12.009] [PMID]
Obsessive-compulsive disorder (OCD) is an important cause of mental health-related morbidity (Fineberg, et al., 2020). World Health Organization (WHO) has classified OCD as one of ten diseases associated with notable disability globally. Its estimated prevalence in the United States is 2. 3% for lifetime OCD and 1. 2% for 12-month criteria. On the other hand, according to a study in six European countries, the lifetime prevalence of OCD in the general population is estimated between 1 and 2%. The prevalence of OCD in Iran is 1. 8% (07% and 28% in men and women, respectively) (Mohammadi, et al.2004). OCD is often associated with severe disruption in family functioning as well as impaired peer relationships and academic performance (Brezinka, Mailänder, & Walitza, 2020). OCD is associated with poor quality of life (Grochtdreis, et al., 2023) and increased risk of mortality (Kim, et al., 2023). Chronic OCD is associated with significant mental and physical morbidity, and in severe cases, it can lead to suicidal behavior, long-term hospitalization, and residential care (Fineberg, Cinosi, Smith, Busby, Wellsted, et al., 2023).
Despite the impact OCD can have on someone’s life, only a minority of those affected seek help from a psychiatrist, with about 30 to 40% receiving assistance (Swierkosz-Lenart, et al., 2023). Research is being prioritized on developing treatments that can improve health outcomes for individuals with OCD (Kammen, et al., 2021; Wang, et al., 2023; Corlier, et al., 2023). Despite receiving adequate treatment, almost 40% of patients do not respond to either treatment. This highlights the importance of developing new treatment methods. Moreover, the use of invasive treatment methods like deep brain stimulation or surgical intervention has yielded limited success (Shivakumar, et al., 2019). Other forms of intervention, such as neuromodulation techniques, are increasingly being studied as a treatment for OCD (Kammen, et al., 2021). The use of electrical currents in the brain is a common technique in neuromodulation therapies, but this current can also regulate local and network-level activities (Borgomaneri, et al., 2021). Studies have shown that approximately two-thirds of people with OCD do not achieve a satisfactory response (Wang, et al., 2023). The current research indicates that non-invasive methods of brain stimulation may be effective in alleviating OCD symptoms (Begemann, Brand, Ćurčić-Blake, Aleman, & Sommer, 2020). Transcranial magnetic stimulation (TMS), transcranial alternating current stimulation (tACS), electroconvulsive therapy (ETC), deep brain stimulation (DBS), and focused ultrasound are examples of interventions that can modify the electrical activity of the brain. These therapies are effective in neurological disorders such as anxiety, depression, and hypertension (Borgomaneri, et al., 2021). Of these, only repetitive transcranial magnetic stimulation (rTMS) and DBS show a high level of evidence in the treatment of OCD (Wang, et al., 2023; Corlier, et al., 2023). Approximately 20 years have been spent exploring the potential of using repetitive TMS as a treatment for OCD (Fitzsimmons et al., 2022).
Currently, leading treatment options for OCD include selective serotonin reuptake inhibitors (SSRIs) and cognitive behavioral therapy (CBT) (Del Casale, et al., 2019; Uhre, et al., 2020). In meta-analysis studies, SSRIs were better tolerated. To be effective, these drugs must be used in appropriate doses and durations (Zhou, et al., 2019). Additionally, meta-analyses have recommended higher doses of SSRIs for the treatment of OCD (Thamby and Jaisoorya, 2019). To evaluate the effectiveness of these drugs and replace or enhance them, the drugs must be used for at least 12 weeks. People who do not respond to one SSRI may respond to another SSRI or enhance their treatment regimen with additional medications. Most evidence-based studies have been conducted to augment first-line medications with low-dose dopamine receptor antagonists (i.e. antipsychotics), some, but not all, have confirmed their effectiveness. Other drugs have also been studied and recommended to enhance the therapeutic effects of SSRIs, including low-dose clomipramine, glutamatergic drugs, and D-cycloserine. However, drug selection in treatment-resistant patients must be based on multiple clinical considerations, including the presence of other comorbidities (Jahanbakhsh, Majidi, & Karimi, 2023). Evidence-based treatments are currently available, mainly involving CBT with exposure and response prevention, or pharmacological treatment with relapse inhibitors. The SSRI often produces disappointing results: Approximately 40% of patients do not respond and 50% require additional treatment (Fineberg, et al., 2020). Chronic OCD is associated with significant mental and physical illness, and in severe cases, it can lead to suicidal behavior, long-term hospitalization, and residential care (Pellegrini, et al., 2021).
Developing new treatments to improve health outcomes for people with OCD is an identified research priority (Fineberg, et al., 2018). Evidence from randomized controlled trials and meta-analyses support its effectiveness and have identified the orbitofrontal cortex (OFC) and supplementary motor area (SMA) as targets promising (Lusicic, et al., 2018). Deep TMS is a form of rTMS that can theoretically regulate deeper subcortical structures. Deep TMS targeting the anterior cingulate cortex is effective for OCD in 2 sham-controlled trials and has received FDA approval (Carmi, et al., 2022; Carmi, et al., 2019). In August 2020, the UK National Institute for Health and Care Excellence (NICE) stipulated that rTMS should only be used in a research context with OCD patients (Pellegrini, et al., 2022). rTMS is also relatively expensive, requires specialized technical equipment and personnel, and cannot be performed in the patient’s home. With the above considerations in mind, the clinical effectiveness of rTMS needs to be evaluated based on its potential to provide OCD patients with a safe and lasting improvement in quality of life. This scoping review identifies what we know and address the gaps in our knowledge in this area to evaluate and provide information on the potential therapeutic effects of rTMS for OCD.
Materials and Methods
The present study was quasi-experimental with a pre-post control group design. The statistical population of the present study included all patients referred to specialized clinics in the second, third, and seventh districts of Tehran City, Iran, from July to September 2022. Participants of the study were 45 OCD patients purposively selected and divided into two experimental groups (rTMS: 15 participants, pharmacotherapy: 15 participants) and a control group with 15 participants. A table of random numbers was used to randomly assign participants into groups. A priori sample size calculations show that, based on a medium effect size (η2=0. 5), a critical p of 0.05, and a critical power level of 0.95, the required sample size is 42. Finally, with the withdrawal of some participants, the estimated sample size was 45.
Patients were eligible if they: (a) were 18 years of age or older; (b) (DSM-5) defined OCD was determined by a research psychiatrist using the Mini International Neuropsychiatric Interview version 7. 02 for DSM-5; (c) duration of symptoms >1 year (based on medical history); and (d) have the Millon clinical multiaxial inventory-III or SCL-90-R obsessive-compulsive scale (OCS), which represents OCD of at least moderate severity.
Patients were excluded if they: (a) were absent from meetings, failed to complete tasks, had unforeseen events (such as illness, death of a relative, etc.), and presented expressed refusal to continue cooperation; (b) have a clinical history of schizophrenia, psychotic symptoms, bipolar disorder, Tourette’s syndrome (not excluding tics not equivalent to Tourette’s syndrome), organic psychosis physical, psychosurgical, borderline disorder or histrionic personality type; (c) had an alcohol or substance use disorder in the past 12 months; (d) have another diagnostic and statistical manual of mental disorder, 5th edition (DSM-5) considered the primary treatment target; or (e) had moderate (20-28) and severe (29-63) depression, as determined by the Beck depression inventory at baseline.
In the research administration, we had to work together with Sajad Hospital and counseling centers (Aram and Rayeheye Omid) in District Three. The researchers worked with the center officials to get the right people for their study. Then they gave them surveys and treatment. It is important to mention that after choosing the people for the study, we explained the treatment plans and goals to them. We also told them that they could choose to participate on their own, and they were not forced to join.
In a group of repetitive magnetic stimulation of the brain from the skull, the duration of sessions followed the following parameters at a rate of three sessions per week for four weeks. The location of coil was placed in the dorsolateral prefrontal cortex (DLPFC) on the left corresponding to point F3 and on the right corresponding to point F4 and SMA area based on the international 10-20 position system for electroencephalography map (EEG). Therefore, rTMS treatment was performed with the parameters specified below regarding the variables in this study. For OCD, the SMA of 1 Hz (HZ) for 20 minutes, 1200 pulses, and areas F3 and F4 were worked with the tablet. Three minutes from zone F3 (intermittent theta bursts) and 40 seconds from zone F4 (cantinu theta burst) after the intervention, people in the control group received treatment as usual.
After the end of the intervention, re-evaluation was done with the tools used in the initial evaluation. The drug interventions during the 4 weeks of the OCD group were: Fluoxetine for the first ten days, one 10 mg capsule in the morning, then one 20 mg capsule in the morning. 2- Tab. Clomipramine 25 mg. For the first ten days, one at night, then one at noon, and one at night. 3- Tab. Clonazepam / 5mg, One Tab every night. All interventions were performed by the first author of the article. Informed consent was obtained from all individual participants included in the present study. According to the results of the Kolmogorov-Smirnov test and the threshold of significance obtained for actual OCD (P=0.145), the data of the variables at two periods are normal. According to the Levine test to verify the homogeneity of variances for OCD (P=0.574) established (P>0.05), and also based on Box’s M test, the hypothesis about the equality of the covariance matrices is approved (Box’s M=0,10, 6.70). Depending on the set of assumptions, the univariate and multivariate covariance analysis for differences between the two groups in the dependent variables. Bonferroni’s post hoc test was adopted to determine the differences between groups. It should be noted that SPSS version 26 software was used for data analysis.
The tools used in this research are as follows:
Millon Clinical Multiaxial Inventory-III (MCMI-III) (Millon, 1994).
The MCMI-III is a test with 175 questions. It is for people 18 and older who can read at an eighth-grade level. The test can be done on paper, on a computer, or online and takes about 25-30 minutes to complete. The test can be scored online, with software, by mail, or by hand. After taking the test, you can get reports that explain your results. The MCMI-III test is very helpful in understanding people’s minds and has been translated into many languages. It has been used in studies that compare different cultures, including Iran (Sharifi, Moulavi, & Namdari, 2008). Problems with understanding MCMI-III include gender, ethnicity, age, and the types of codes used. The MCMI-III test has three parts and 24 different scales. It is based on Millon’s theory of personality and is similar to the DSM-III and DSM-IV diagnostic categories. The scales measure how much people reveal about themselves, how much they want to be liked, and how much they try to make themselves look bad. The clinical scales measure different levels of personality problems, like moderate and severe. The MCMI-III was picked because it is a trustworthy and accurate way to measure personality disorders. As the manual suggests, it uses a cutoff score of 85 or higher to meet the diagnostic criteria. The Persian version of the MCMI-III test was very accurate. It was tested and proved reliable (Sharifi, et al., 2008).
Symptom Checklist-90-Revision (SCL-90-R)
SCL-90-R is a multidimensional self-report symptom inventory and its derived Iranian standard version was used in this study (Alavi, et al., 2012). The SCL-90-R has 90 questions divided into nine categories: Physical problems, obsessive thoughts, sensitivity to others, feeling sad, feeling worried, anger, fear, thinking others are out to harm you, and feeling disconnected from reality. The nine different symptoms were grouped into three big categories: The global severity index shows how serious the psychiatric problem is, the positive symptom total measures how many questions were rated as a problem, and the positive symptom distress index shows how intense the symptoms are. In this research, the Iranian version of SCL-90-R was found to be reliable with a Cronbach alpha of 0. 95 and split-half reliability of 0. 88.
Results
The mean±SD age of patients in the experimental group was 45.60±0.67 years and that of the control group was 45.46±0.67 years. According to the findings, the three groups in this research are almost homogeneous in the variables of age, education, gender, and marital status. The results of the chi-square test also show that the differences between the three groups in terms of age (P=0.861), education (P=0.685), gender (P=0.433), and marital status (P=0.738) are not significant (P>0.05).
As seen, the mean in the magnetic stimulation group from the skull and the drug therapy group in the post-test stage decreases compared to the pre-test. Based on the results recorded in Table 1, we can describe that magnetic stimulation treatments from the skull and drug therapy have reduced the obsessive-compulsive components of the patients.

According to the results of significant levels obtained for OCD, the Kolmogorov Smirnov test (data normality test (P=0.145), which is greater than 0.05, and the data of the variables in both stages are normal and can be tested using parametric tests. As can be seen, the assumption of equality of variances for OCD (P=0.574) is established (P>0.05). The standard method for evaluating the equality of covariance matrices, the M box test is used where a significant level less than 0.05 is considered as an index of heterogeneity or inequality. As can be seen, the assumption of equality of covariance matrices is maintained (M box=0.10, 6.70).
The results in Table 2 show that by removing the effects of pre-test variables and considering the calculated F-factors, there is a significant difference between the adjusted means of participants’ obsessive-compulsive scores in the post-test phase (F=458.11 P= 0.0001).

According to Table 3, the consequences of the study reasoned that the differentiation between the treatment technique of rTMS (P=0.0001) and drug administration (P=0.0001) is significant in OCD. The decrease in OCD based on rTMS was more than the drug therapy group (P=0.0017).

Discussion
The study investigated the comparison of the effectiveness of rTMS and drug administration in reducing OCD symptoms. The consequences of the study reasoned that the differentiation between the treatment technique for rTMS and drug administration is significant in OCD. The reduction in Yale-Brown obsessive–compulsive scale (YBOCS) score was significantly greater at the post-treatment assessment. In other words, in rTMS, the OCD score decreased more than in the drug treatment group. Consistent with our findings, Adu et al. (2021) found using rTMS on specific areas of the brain is both effective and well-tolerated for OCD treatment. This is especially true for the SMA and the OFC, compared to other areas like the prefrontal cortex. Although the studies varied in their results and how severe the symptoms were, rTMS seems like a good treatment for OCD. Furthermore, using low-frequency rTMS over the left dorsolateral prefrontal cortex (DLPFC) can help reduce Y-BOCS scores in OCD patients who have not responded to other treatments. This effect lasts for at least three to six months after 15 sessions of treatment. Using both antipsychotic and serotonergic medications along with rTMS therapy may help predict how well the adjunctive treatment will work (Jahanbakhsh, Majidi, & Karimi, 2023). Using high-frequency magnetic stimulation over specific parts of the brain can help improve symptoms of OCD. This could be a helpful option for people who do not see good results from medication or therapy (Carmi, et al., 2022). In recent years, several attempts to treat OCD with rTMS have been reported (Uhre, et al., 2020; Kammen, et al., 2021; Wang, et al., 2023), and a recent meta-analysis found that rTMS performed clinically and statistically superior to sham treatment (Fitzsimmons et al., 2022).
Recently, it was found that doing deep transcranial magnetic stimulation therapy for six weeks every day is safe and works well for people with OCD who do not get better with medicine or CBT (Carmi et al., 2018, 2019). Two studies recently looked at how often and where rTMS is used for OCD (Liang et al., 2021; Perera et al., 2021). They came to different conclusions about how well it works. One study found that stimulating both sides of the brain is the best treatment option (Perera et al., 2021), while another study found that stimulating the brain at a low frequency is the most effective treatment (Liang et al., 2021). This difference in interpretation may be due to the decision of the two studies not to separate and distinguish between unique frequency and location combinations. Classically, high-frequency rTMS and intermittent theta burst stimulation (iTBS) are thought to increase cortical excitability, whereas low-frequency rTMS and continuous theta burst stimulation (cTBS) are thought to causing inhibition of underlying brain tissue (Rossi et al., 2009). In the context of depression treatment, stimulation of the left or right DLPFC with high-frequency versus low-frequency rTMS may have different therapeutic effects (Brunoni et al., 2017). This indicates that frequency and location have an important and independent influence on the effectiveness of rTMS and therefore should be considered separately when evaluating and comparing different protocols.
The cause of OCD, according to brain scans, is related to a problem in a circuit of brain areas including the OFC, DLPFC, and thalamus. Changing the way the brain works with surgery and deep brain stimulation helps lessen symptoms of OCD. Considering the chance that rTMS can change parts of the brain, researchers have looked at how it could help treat OCD by targeting specific brain circuits. They have found that rTMS may help balance out abnormal brain activity in people with OCD (Adu, et al., 2021).
A lot of evidence shows that deep rTMS therapy works well for OCD, as used in the United States. The treatment will probably be used more by doctors if it is approved by the FDA. In this new report, rTMS was used because the patient had a history of reversible cerebral vasoconstriction syndrome (RCVS) with bleeding inside the head, possibly caused by fluvoxamine. The use of birth control pills at the same time might have also played a part in this. (Silveira, Damiano, Abelama Neto, Gomes, et al., 2022). In a group of 210 patients who had not started using SSRI again within 6 months to 7 years, RCVS came back in 5% of the patients in a study by Chen, et al. (2015). We do not know if starting SSRI again increases the risk of RCVS coming back. It is really important to study this and find other treatments because RCVS is very serious. In addition to causing RCVS, SSRI medicines have also been linked to a small increase in the chance of bleeding in the brain (Douros, Ades, & Renoux, 2018).
In a 2020 cohort of 1279 patients with retrocranial hemorrhage, a higher rate of recurrent intracranial hemorrhage was observed in SSRI users, and this association was greater in patients at risk of hemorrhage. Postcranial blood is higher in the ɛ4 allele, in patients with lobar intracranial hemorrhage, and patients with previous intracranial hemorrhage (Kubiszewski, et al., 2021). Researchers looked at 55 experiments testing 19 different treatments or fake treatments, with a total of 2011 people involved. Ondansetron, deep TMS, therapist-administered cognitive behavioral therapy (CBT-TA), and aripiprazole were rated as the top four treatments based on their surface under the cumulative ranking percentages. Although all four treatments had a big impact, CBT[TA] came very close to being significant but just missed it. In studies, deep TMS was found the most effective treatment for OCD in people who did not respond to serotonin reuptake inhibitor medications (Suhas, et al 2022).
Conclusion
This study revealed that rTMS is an effective neuro-stimulation therapy for OCD. Therefore, rTMS provides better results in terms of treatment effectiveness and clinical response rate. Furthermore, drug treatment appears to have a significant therapeutic effect. In subgroup analysis, it was found that DLPFC stimulation and inhibition produced better treatment effects. Larger follow-up periods and sample sizes are needed for future randomized controlled trials to study better protocols.
Limitations
There are several shortcomings of our study that need to be addressed. First, it was conducted at a tertiary care psychology center, which preferentially recruited patients with more severe levels of OCD. Second, despite calculating an adequate sample size, our sample can still be considered small, generating large confidence intervals, thus necessitating replication of our results in larger samples. Third, lack of follow-up time. Further work is needed to better evaluate the effectiveness and safety of this rTMS protocol, as well as its individualization. Therefore, rTMS may be an important alternative treatment for these individuals as well as for those with other contraindications to SSRIs.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in studies involving human participants were by the ethical standards of the institutional and or national Research Committee by the code IR.IAU.SEMNAN.REC.1402.022.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Investigation, data collection, data analysis: Hooshang Esmaili; Writing-original draft, Methodology: Hooshang Esmaili; Conceptualization and Supervision: Nemat Sotoudeh Asl; Writing–review & editing: Nemat Sotoudeh Asl and Raheb Ghornabni.
Conflict of interest
The authors declare no conflict of interest.
References
Adu, M. K., Eboreime, E., Sapara, A. O., Greenshaw, A. J., Chue, P., & Agyapong, V. I. O. (2021). The use of repetitive transcranial magnetic stimulation for treatment of obsessive-compulsive disorder: A scoping review. Mental Illness, 13(1), 1–13.[DOI:10.1108/MIJ-05-2021-0002] [PMID]
Alavi, S. S., Alaghemandan, H., Maracy, M. R., Jannatifard, F., Eslami, M., & Ferdosi, M. (2012). Impact of addiction to internet on a number of psychiatric symptoms in students of isfahan universities, iran, 2010. International Journal of Preventive Medicine, 3(2), 122–127. [PMID] [PMCID]
Begemann, M. J., Brand, B. A., Ćurčić-Blake, B., Aleman, A., & Sommer, I. E. (2020). Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: A meta-analysis. Psychological Medicine, 50(15), 2465–2486. [DOI:10.1017/S0033291720003670] [PMID]
Borgomaneri, S., Battaglia, S., Sciamanna, G., Tortora, F., & Laricchiuta, D. (2021). Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neuroscience and Biobehavioral Reviews, 127, 334–352. [DOI:10.1016/j.neubiorev.2021.04.036] [PMID]
Brezinka, V., Mailänder, V., & Walitza, S. (2020). Obsessive compulsive disorder in very young children - a case series from a specialized outpatient clinic. BMC Psychiatry, 20(1), 366. [DOI:10.1186/s12888-020-02780-0] [PMID]
Brunoni, A. R., Chaimani, A., Moffa, A. H., Razza, L. B., Gattaz, W. F., & Daskalakis, Z. J., et al. (2017). Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: A systematic review with network meta-analysis. JAMA Psychiatry, 74(2), 143–152. [DOI:10.1001/jamapsychiatry.2016.3644] [PMID]
Carmi, L., Tendler, A., Bystritsky, A., Hollander, E., Blumberger, D. M., Daskalakis, J., ... & Zohar, J. (2019). Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. American Journal of Psychiatry, 176(11), 931-938. [DOI:10.1176/appi.ajp.2019.18101180] [PMID]
Carmi, L., Tendler, A., Bystritsky, A., Hollander, E., Blumberger, D. M., & Daskalakis, J., et al. (2022). Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: A prospective multicenter randomized double-blind placebo-controlled trial. Focus (American Psychiatric Publishing), 20(1), 152–159. [DOI:10.1176/appi.focus.20103] [PMID]
Chen, S. P., Fuh, J. L., Lirng, J. F., Wang, Y. F., & Wang, S. J. (2015). Recurrence of reversible cerebral vasoconstriction syndrome: A long-term follow-up study. Neurology, 84(15), 1552–1558. [DOI:10.1212/WNL.0000000000001473] [PMID]
Corlier, J., Tadayonnejad, R., Wilson, A. C., Lee, J. C., Marder, K. G., & Ginder, N. D., et al. (2023). Repetitive transcranial magnetic stimulation treatment of major depressive disorder and comorbid chronic pain: Response rates and neurophysiologic biomarkers. Psychological Medicine, 53(3), 823–832. [DOI:10.1017/S0033291721002178] [PMID]
Ferracuti, S., Lamis, D. A., Rapinesi, C., Sani, G., Girardi, P., & Kotzalidis, G. D., et al. (2019). Psychopharmacological treatment of obsessive-compulsive disorder (OCD). Current Neuropharmacology, 17(8), 710–736. [DOI:10.2174/1570159X16666180813155017] [PMID]
Douros, A., Ades, M., & Renoux, C. (2018). Risk of intracranial hemorrhage associated with the use of antidepressants inhibiting serotonin reuptake: A systematic review. CNS Drugs, 32(4), 321–334. [DOI:10.1007/s40263-018-0507-7] [PMID]
Fineberg, N. A., Cinosi, E., Smith, M. V. A., Busby, A. D., Wellsted, D., & Huneke, N. T. M., et al. (2023). Feasibility, acceptability and practicality of transcranial stimulation in obsessive compulsive symptoms (FEATSOCS): A randomised controlled crossover trial. Comprehensive Psychiatry, 122, 152371. [DOI:10.1016/j.comppsych.2023.152371] [PMID]
Fineberg, N. A., Demetrovics, Z., Stein, D. J., Ioannidis, K., Potenza, M. N., & Grünblatt, E., et al. (2018). Manifesto for a European research network into Problematic Usage of the Internet. European Neuropsychopharmacology, 28(11), 1232–1246. [DOI:10.1016/j.euroneuro.2018.08.004] [PMID]
Fineberg, N. A., Hollander, E., Pallanti, S., Walitza, S., Grünblatt, E., & Dell'Osso, B. M., et al. (2020). Clinical advances in obsessive-compulsive disorder: A position statement by the International College of Obsessive-Compulsive Spectrum Disorders. International Clinical Psychopharmacology, 35(4), 173–193. [DOI:10.1097/ YIC.0000000000000314] [PMID]
Fitzsimmons, S. M. D. D., van der Werf, Y. D., van Campen, A. D., Arns, M., Sack, A. T., & Hoogendoorn, A. W., et al. (2022). Repetitive transcranial magnetic stimulation for obsessive-compulsive disorder: A systematic review and pairwise/network meta-analysis. Journal of Affective Disorders, 302, 302–312. [DOI:10.1016/j.jad.2022.01.048] [PMID]
Grochtdreis, T., König, H. H., Gallinat, J., Konnopka, A., Schulz, H., & Lambert, M., et al. (2023). Validation of the recovering quality of life (ReQoL) questionnaires for patients with anxiety, obsessive-compulsive, stress-related, somatoform and personality disorders in Germany. Journal of Psychiatric Research, 157, 202–211. [DOI:10.1016/j.jpsychires.2022.11.032] [PMID]
Jahanbakhsh, G., Alireza Haji Seyed Javadi, S., Majidi, M., Khademi, M., & Karimi, R. (2023). Effectiveness of adjunctive low-frequency repetitive transcranial magnetic stimulation therapy over the left dorsolateral prefrontal cortex in patients with obsessive-compulsive disorder refractory to medical treatment: A double-blind, randomized clinical trial. Asian Journal of Psychiatry, 80, 103384. [DOI:10.1016/j.ajp.2022.103384] [PMID]
Kammen, A., Cavaleri, J., Lam, J., Frank, A. C., Mason, X., & Choi, W., et al. (2022). Neuromodulation of OCD: A review of invasive and non-invasive methods. Frontiers in Neurology, 13, 909264. [DOI:10.3389/fneur.2022.909264] [PMID]
Kim, H., Kim, S. H., Jeong, W., Park, Y. S., Kim, J., & Park, E. C., et al. (2023). Association between obsessive-compulsive disorder and the risk of schizophrenia using the Korean National Health Insurance Service-National Sample Cohort: A retrospective cohort study. Epidemiology and Psychiatric Sciences, 32, e9. [DOI:10.1017/S2045796023000021] [PMID]
Kubiszewski, P., Sugita, L., Kourkoulis, C., DiPucchio, Z., Schwab, K., & Anderson, C. D., et al. (2020). Association of selective serotonin reuptake inhibitor use after intracerebral hemorrhage with hemorrhage recurrence and depression severity. JAMA Neurology, 78(1), 1–8. [DOI:10.1001/jamaneurol.2020.3142] [PMID]
Liang, K., Li, H., Bu, X., Li, X., Cao, L., & Liu, J., et al. (2021). Efficacy and tolerability of repetitive transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder in adults: A systematic review and network meta-analysis. Translational Psychiatry, 11(1), 332. [DOI:10.1038/s41398-021-01453-0] [PMID]
Lusicic, A., Schruers, K. R., Pallanti, S., & Castle, D. J. (2018). Transcranial magnetic stimulation in the treatment of obsessive-compulsive disorder: Current perspectives. Neuropsychiatric Disease and Treatment, 14, 1721–1736. [DOI:10.2147/NDT.S121140] [PMID]
Mohammadi, M. R., Ghanizadeh, A., Rahgozar, M., Noorbala, A. A., Davidian, H., & Afzali, H. M., et al. (2004). Prevalence of obsessive-compulsive disorder in Iran. BMC Psychiatry, 4, 2. [DOI:10.1186/1471-244X-4-2] [PMID]
Pellegrini, L., Garg, K., Enara, A., Gottlieb, D. S., Wellsted, D., & Albert, U., et al. (2022). Repetitive transcranial magnetic stimulation (r-TMS) and selective serotonin reuptake inhibitor-resistance in obsessive-compulsive disorder: A meta-analysis and clinical implications. Comprehensive Psychiatry, 118, 152339. [DOI:10.1016/j.comppsych.2022.152339] [PMID]
Pellegrini, L., Maietti, E., Rucci, P., Burato, S., Menchetti, M., & Berardi, D., et al. (2021). Suicidality in patients with obsessive-compulsive and related disorders (OCRDs): A meta-analysis. Comprehensive Psychiatry, 108, 152246. [DOI:10.1016/j.comppsych.2021.152246] [PMID]
Perera, M. P. N., Mallawaarachchi, S., Miljevic, A., Bailey, N. W., Herring, S. E., & Fitzgerald, P. B. (2021). Repetitive transcranial magnetic stimulation for obsessive-compulsive disorder: A meta-analysis of randomized, sham-controlled trials. biological psychiatry. Cognitive Neuroscience and Neuroimaging, 6(10), 947–960. [DOI:10.1016/j.bpsc.2021.03.010] [PMID]
Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A., & Safety of TMS Consensus Group (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. [DOI:10.1016/j.clinph.2009.08.016] [PMID]
Sharifi, A. A., Moulavi, H., & Namdari, K. (2008). [The validity of MCMI-III (Millon, 1994) scales (Persian)]. Knowledge & Research in Psychology, 34, 27-38. [Link]
Shivakumar, V., Dinakaran, D., Narayanaswamy, J. C., & Venkatasubramanian, G. (2019). Noninvasive brain stimulation in obsessive-compulsive disorder. Indian Journal of Psychiatry, 61(Suppl 1), S66–S76. [DOI:10.4103/psychiatry.IndianJPsychiatry_522_18] [PMID]
Silveira, J. B., Damiano, R. F., Abelama Neto, E., Gomes, R. N. D. S. M., Klein, I., & Borrione, L., et al. (2022). Double cone coil repetitive transcranial magnetic stimulation for severe obsessive-compulsive disorder after reversible cerebral vasoconstriction syndrome with intracerebral hemorrhage: A case report. Brazilian Journal of Psychiatry, 44(5), 562-564. [DOI:10.47626/1516-4446-2022-2556]
Suhas, S., Malo, P. K., Kumar, V., Issac, T. G., Chithra, N. K., & Bhaskarapillai, B., et al. (2023). Treatment strategies for serotonin reuptake inhibitor-resistant obsessive-compulsive disorder: A network meta-analysis of randomised controlled trials. The World Journal of Biological psychiatry, 24(2), 162–177. [DOI:10.1080/15622975.2022.2082525] [PMID]
Swierkosz-Lenart, K., Dos Santos, J. F. A., Elowe, J., Clair, A. H., Bally, J. F., & Riquier, F., et al. (2023). Therapies for obsessive-compulsive disorder: Current state of the art and perspectives for approaching treatment-resistant patients. Frontiers in Psychiatry, 14, 1065812. [DOI:10.3389/fpsyt.2023.1065812] [PMID]
Thamby, A., & Jaisoorya, T. S. (2019). Antipsychotic augmentation in the treatment of obsessive-compulsive disorder. Indian Journal of Psychiatry, 61(Suppl 1), S51–S57. [DOI:10.4103/psychiatry.IndianJPsychiatry_519_18] [PMID]
Uhre, C. F., Uhre, V. F., Lønfeldt, N. N., Pretzmann, L., Vangkilde, S., Plessen, K. J., ... & Pagsberg, A. K. (2020). Systematic review and meta-analysis: cognitive-behavioral therapy for obsessive-compulsive disorder in children and adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 59(1), 64-77. [DOI:10.1016/j.jaac.2019.08.480] [PMID]
Wang, J., Hua, G., Wang, S., Guo, G., Quan, D., & Yao, S., et al. (2023). Glutamatergic neurotransmission is affected by low-frequency repetitive transcranial magnetic stimulation over the supplemental motor cortex of patients with obsessive-compulsive disorder. Journal of Affective Disorders, 325, 762–769. [DOI:10.1016/j.jad.2023.01.064] [PMID]
Zhou, D. D., Zhou, X. X., Li, Y., Zhang, K. F., Lv, Z., & Chen, et al. (2019). Augmentation agents to serotonin reuptake inhibitors for treatment-resistant obsessive-compulsive disorder: A network meta-analysis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 90, 277–287. [DOI:10.1016/j.pnpbp.2018.12.009] [PMID]
Type of Study: Research |
Subject:
Rehabilitation
Received: 2023/04/8 | Accepted: 2023/08/30 | Published: 2024/01/14
Received: 2023/04/8 | Accepted: 2023/08/30 | Published: 2024/01/14
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |